Ice VII
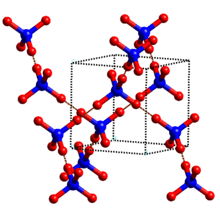
Ice VII is a cubic crystalline form of ice. It can be formed from liquid water above 3 GPa (30,000 atmospheres) by lowering its temperature to room temperature, or by decompressing heavy water (D2O) ice VI below 95 K. Ordinary water ice is known as ice Ih, (in the Bridgman nomenclature). Different types of ice, from ice II to ice XVI, have been created in the laboratory at different temperatures and pressures. Ice VII is metastable over a wide range of temperatures and pressures and transforms into low density amorphous ice (LDA) above 120K.[1] Ice VII has a triple point with liquid water and ice VI at 355 K and 2.216 GPa, with the melt line extending to at least 715 K and 10 GPa.[2] It can also be created by increasing the pressure on ice VI at ambient temperature.[3]
Like the majority of ice phases (including ice Ih), the hydrogen atom positions are disordered.[4] In addition, the oxygen atoms are disordered over multiple sites.[5][6][7] The structure of ice VII comprises a hydrogen bond framework in the form of two interpenetrating (but non-bonded) sub-lattices.[5] Hydrogen pass through the center of the water hexamers and thus do not connect the two lattices. Ice VII has a density of about 1.65 g cm−3 (at 2.5 GPa and 25 °C),[8] which is less than twice the cubic ice density as the intra-network O····O distances are 8% longer (at 0.1 MPa) to allow for interpenetration. The cubic unit cell has a side length of 3.3501 Å (for D2O, at 2.6 GPa and 22 °C) and contains two water molecules.[6]
Ice VII is the only disordered phase of ice that can be ordered by simple cooling,[3][9] and it forms (ordered) ice VIII below 273 K up to ~ 8 GPa. Above this pressure, the VII-VIII transition temperature drops rapidly, reaching 0 K at ~60 GPa.[10] Thus, ice VII has the largest stability field of all of the molecular phases of ice. The cubic oxygen sub-lattices that form the backbone of the ice VII structure persist to pressures of at least 128 GPa;[11] this pressure is substantially higher than that at which water loses its molecular character entirely, forming ice X. In high pressure ices, protonic diffusion (movement of protons around the oxygen lattice) dominates molecular diffusion, an effect which has been measured directly.[12]
Scientists hypothesize that ice VII may comprise the ocean floor of Titan as well as extrasolar planets (such as Gliese 436 b and GJ 1214 b) that are largely made of water.[13][14]
References
- Chaplin, Martin (2007-10-26). "Ice-seven and ice-ten structures". Water Structure and Science. Retrieved 2008-01-02.
- ↑ S. Klotz, J. M. Besson, G. Hamel, R. J. Nelmes, J. S. Loveday and W. G. Marshall, Metastable ice VII at low temperature and ambient pressure, Nature 398 (1999) 681-684.
- ↑ "IAPWS, Release on the pressure along the melting and the sublimation curves of ordinary water substance, 1993" (PDF). Retrieved 2008-02-22.
- 1 2 Johari, G. P.; Lavergne, A. & Whalley, E. (1974), "Dielectric properties of ice VII and VIII and the phase boundary between ice VI and VII", Journal of Chemical Physics, 61 (10): 4292, Bibcode:1974JChPh..61.4292J, doi:10.1063/1.1681733
- ↑ Petrenko, V. F.; Whitworth, R. W. (2002), The Physics of Ice, New York: Oxford University Press.
- 1 2 Kuhs, W. F.; Finney, J. L.; Vettier, C. & Bliss, D. V. (1984), "Structure and hydrogen ordering in ices VI, VII, and VIII by neutron powder diffraction", Journal of Chemical Physics, 81 (8): 3612–3623, Bibcode:1984JChPh..81.3612K, doi:10.1063/1.448109.
- 1 2 Jorgensen, J. D.; Worlton, T. G. (1985), "Disordered structure of D2O ice VII from in situ neutron powder diffraction", Journal of Chemical Physics, 83 (1): 329–333, Bibcode:1985JChPh..83..329J, doi:10.1063/1.449867.
- ↑ Nelmes, R. J.; Loveday, J. S.; Marshall, W. G.; et al. (1998), "Multisite Disordered Structure of Ice VII to 20 GPa", Physical Review Letters, 81 (13): 2719–2722, Bibcode:1998PhRvL..81.2719N, doi:10.1103/PhysRevLett.81.2719.
- ↑ D. Eisenberg and W. Kauzmann, The structure and properties of water (Oxford University Press, London, 1969); (b) The dodecahedral interstitial model is described in L. Pauling, The structure of water, In Hydrogen bonding, Ed. D. Hadzi and H. W. Thompson (Pergamon Press Ltd, London, 1959) pp 1-6.
- ↑ Note: ice Ih theoretically transforms into proton-ordered ice XI on geologic timescales, but in practice it is necessary to add small amounts of KOH catalyst.
- ↑ Pruzan, Ph.; Chervin, J. C. & Canny, B. (1993), "Stability domain of the ice VIII proton-ordered phase at very high pressure and low temperature", Journal of Chemical Physics, 99 (12): 9842–9846, Bibcode:1993JChPh..99.9842P, doi:10.1063/1.465467.
- ↑ Hemley, R. J.; Jephcoat, A. P.; Mao, H. K.; et al. (1987), "Static compression of H2O-ice to 128 GPa (1.28 Mbar)", Nature, 330 (6150): 737–740, Bibcode:1987Natur.330..737H, doi:10.1038/330737a0.
- ↑ Katoh, E. (15 February 2002). "Protonic Diffusion in High-Pressure Ice VII". Science. 295 (5558): 1264–1266. Bibcode:2002Sci...295.1264K. doi:10.1126/science.1067746.
- ↑ University of Liège (2007, May 16). Astronomers Detect Shadow Of Water World In Front Of Nearby Star. ScienceDaily. Retrieved Jan. 3, 2010, from http://www.sciencedaily.com/releases/2007/05/070516151053.htm
- ↑ David A. Aguilar (2009-12-16). "Astronomers Find Super-Earth Using Amateur, Off-the-Shelf Technology". Harvard-Smithsonian Center for Astrophysics. Retrieved January 23, 2010.