IFNA2
| View/Edit Human | |||
Interferon alpha-2 is a protein that in humans is encoded by the IFNA2 gene.[2]
Interferon α2 within its family
Human interferon alpha-2 (IFNα2) is a cytokine belonging to the family of type I IFNs. IFNα2 is a protein secreted by cells infected by a virus and acting on other cells to inhibit viral infection. The first description of IFNs as a cellular agent interfering with viral replication was made by Alick Isaacs and Jean Lindenmann in 1957. The history of this finding was recently reviewed.[3] There are 3 types of IFNs: Interferon type I, Interferon type II and Interferon type III. The type II IFN, also called IFNγ, is produced by specific cells of the immune system. Unlike type I and type III IFNs, IFNγ has only a modest role in directly restricting viral infections. Type I and type III IFNs act similarly. However, the action of type III IFNs, also known as IFNλ, is limited to epithelial cells while type I IFNs act on all body's cells.
Type I IFNs form a family of several proteins: in humans, there are 13 α subtypes, 1 β subtype, 1 ω subtype and other less studied subtypes (κ and ε).[4] IFNα2 was the first subtype to be characterized in the early eighties. As a result, IFNα2 was widely used in basic research to elucidate biological activities, structure and mechanism of action of type I IFNs. IFNα2 was also the first IFN to be produced by the pharmaceutical industry for use as a drug. Thereby, IFNα2 is the best known type I IFN subtype. The properties of IFNα2 are widely shared by the other type I IFNs, although subtle differences exist.
IFNα2: from gene to protein
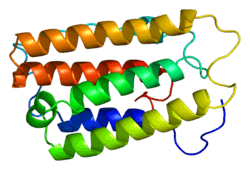
The gene encoding IFNα2, the IFNA2 gene, is clustered with all other type I IFN genes on chromosome 9 [5] and as all type I IFN genes, it is devoid of intron.[6] The open reading frame (coding sequence) of IFNA2 codes for a pre-protein of 188 amino acids with a 23 amino acid signal peptide allowing secretion of the mature protein. The mature protein is made of 165 amino acids, one less than the other human IFNα subtypes. The secondary structure of IFNα2 consists of five α-helices: A to E, from the N-terminal to the C-terminal end. Helices A, B, C and E are organized as a bundle with a long loop between the helices A and B (the A-B loop) and two disulfide bonds which connect helix E to the A-B loop and helix C to the N-terminal end.[7][8] Several variants, or allelic variants, have been identified in the human population.[9] Among them, IFNα2a and IFNα2b are better known by their commercial name, Roferon-A® and Intron A®, respectively. Upstream of the coding sequence is the promoter region that contains sequences that regulate the transcription of the IFNA2 gene into a messenger RNA (mRNA).[10][11]
The production of type I IFNs
When a cell is infected by a virus, some components of the virus, mainly viral nucleic acids, are recognized by specialized cellular molecules such as RIG-I, MDA5 and some toll-like receptors (TLR).[12] This recognition induces the activation of specific serine kinases, enzymes which activate by phosphorylation the IFN regulatory factors (IRF), IRF3 and IRF7. IRF3 and IRF7 are themselves transcription factors that translocate into the nucleus and activate the transcription of type I IFNs genes and thereby initiate the process leading to the secretion of IFN by the infected cells. The "danger" signals carried by viruses were the first IFN inducers described but it is now known that non-viral "danger" signals, such as some types of dead cells, can stimulate the synthesis of type I IFNs.
Mechanism of action
Induced IFNα2 is secreted by the infected cells and acts locally as well as systemically on cells expressing a specific cell surface receptor able to bind type I IFNs. The type I IFN receptor (IFNAR) is composed of two subunits, IFNAR 1 and IFNAR 2, which are expressed by all body’s cells. After binding to its receptor,[13] type I IFNs activate multiple cellular factors that transduce the signal from the cell surface into the nucleus.[14] The main signaling pathway activated by type I IFNs consists of a series of events:[15]
- phosphorylation and activation of two enzymes of the Janus kinases or JAK family, TYK2 which is associated with IFNAR1 and JAK1 associated to IFNAR2;
- phosphorylation by the activated JAK kinases of key transcription factors, namely STAT1 and STAT2, members of the family Signal Transducer and Activator of Transcription (STAT protein);
- phosphorylated STAT1 and STAT2 bind IRF9 forming a complex named "IFN-Stimulated Gene Factor 3" (ISGF3). This complex translocates in the nucleus and initiates the transcription of the IFN-stimulated genes (ISGs).
ISGs encode proteins that modulate cellular functions. Following viral infection, many ISGs lead to the inhibition of the viral spread.[12] Several ISGs inhibit viral replication in the infected cells. Other ISGs protect neighbouring uninfected cells from being infected by inhibiting viral entry. Several hundreds of ISGs are known to be activated by type I IFNs [16] and are listed in a searchable database named interferome (http://www.interferome.org/).
Biological activities
The broad spectrum of ISGs explains the wide range of biological activity of type I IFNs.[12][17][18][19][20] In addition to their antiviral activity, type I IFNs also inhibit the proliferation of cells and regulate the activation of the immune system.
Type I IFNs exert potent antitumor activity by several mechanisms such as:
- inhibition of the proliferation of cancer cells
- activation of the immune system which can eliminate tumor cells [21][22]
- increasing the antitumor activity of other antitumoral agents (radiotherapy, chemotherapy, targeted therapies) [23][24][25]
Type I IFNs can have detrimental effects during viral and non-viral infections (bacterial, parasitic, fungal). This is due in part by the ability of type I IFNs to polarize the immune system towards a specific type of response in order to interfere with virus infections.
When improperly regulated, IFN production or IFN-induced signalling can result in autoimmune diseases, such as systemic lupus erythematosus.[26]
Clinical use
If given orally, IFNα2 is degraded by digestive enzymes and is no longer active. Thus, IFNα2 is mainly administrated by injection essentially subcutaneous or intramuscular. Once in the blood, IFNα2 is rapidly eliminated by the kidney. Due to the short life of IFNα2 in the organism, several injections per week are required. Peginterferon alpha-2a and Peginterferon alpha-2b (polyethylene glycol linked to IFNα2) are long-lasting IFNα2 formulations, which enable a single injection per week.
Recombinant IFNα2 (α2a and α2b) has demonstrated efficiency in the treatment of patients diagnosed with some viral infections (such as chronic viral hepatitis B and hepatitis C) or some kinds of cancer (melanoma, renal cell carcinoma and various hematological malignancies).[27] Yet, patients on therapy with IFNα2 suffer from adverse effects which often require to reduce or even stop the treatment.[28] These adverse effects include flu-like symptoms such as chills, fever, joint and muscle pain, depression with suicidal ideation, and a reduction in the number of blood cells. Thereby, IFNα2 has been progressively replaced by better tolerated drugs, such as antiviral agents or targeted antitumor therapies. Chronic viral hepatitis C is the main indication for which IFNα2 remains widely used.[27] Nevertheless, there is increasing evidence that endogenous type I IFNs plays a role in the induction of an immune antiviral response and that they can enhance the antitumor activity of chemotherapies, radiotherapies and some targeted therapies.[23][24][25] Therefore, an important future goal for scientists is to modify IFNα2 in order to obtain an active molecule to be used in the clinic that does not exert adverse effects.[29]
References
- ↑ "Human PubMed Reference:".
- ↑ "Entrez Gene: IFNA2 interferon, alpha 2".
- ↑ Gresser I (2015). "On intuition and the discovery of interferon". Cytokine Growth Factor Rev. In press: 99–101. doi:10.1016/j.cytogfr.2014.11.006. PMID 25547990.
- ↑ Pestka S, Krause CD, Walter MR (Dec 2004). "Interferons, interferon-like cytokines, and their receptors". Immunol Rev. 202: 8–32. doi:10.1111/j.0105-2896.2004.00204.x. PMID 15546383.
- ↑ Díaz MO, Pomykala HM, Bohlander SK, Maltepe E, Malik K, Brownstein B, Olopade OI (Aug 1994). "Structure of the human type-I interferon gene cluster determined from a YAC clone contig". Genomics. 22 (3): 540–52. doi:10.1006/geno.1994.1427. PMID 8001965.
- ↑ Qi Z, Nie P, Secombes CJ, Zou J (May 2010). "Intron-containing type I and type III IFN coexist in amphibians: refuting the concept that a retroposition event gave rise to type I IFNs.". J Immunol. 184 (9): 5038–46. doi:10.4049/jimmunol.0903374. PMID 20357248.
- ↑ Klaus W, Gsell B, Labhardt AM, Wipf B, Senn H (Dec 1997). "The three-dimensional high resolution structure of human interferon alpha-2a determined by heteronuclear NMR spectroscopy in solution". J Mol Biol. 274 (4): 661–75. doi:10.1006/jmbi.1997.1396. PMID 9417943.
- ↑ Radhakrishnan R, Walter LJ, Hruza A, Reichert P, Trotta PP, Nagabhushan TL, Walter MR (Dec 1996). "Zinc mediated dimer of human interferon-alpha 2b revealed by X-ray crystallography". Structure. 412 (12): 1453–63. doi:10.1016/s0969-2126(96)00152-9. PMID 8994971.
- ↑ von Gabain A, Lundgren E, Ohlsson M, Holmgren E, Josephsson S, Alkan SS (Jun 1990). "Three human interferon-alpha 2 subvariants disclose structural and functional differences". Eur J Biochem. 190 (2): 257–61. doi:10.1111/j.1432-1033.1990.tb15570.x. PMID 1694761.
- ↑ Génin P, Lin R, Hiscott J, Civas A (Jun 2009). "Differential regulation of human interferon A gene expression by interferon regulatory factors 3 and 7". Mol Cell Biol. 29 (12): 3435–50. doi:10.1128/MCB.01805-08. PMC 2698742
 . PMID 19349300.
. PMID 19349300. - ↑ Honda K, Yanai H, Takaoka A, Taniguchi T (Nov 2005). "Regulation of the type I IFN induction: a current view". Int Immunol. 17 (11): 1367–78. doi:10.1093/intimm/dxh318. PMID 16214811.
- 1 2 3 Tomasello E, Pollet E, Vu Manh TP, Uzé G, Dalod M (Oct 2014). "Harnessing Mechanistic Knowledge on Beneficial Versus Deleterious IFN-I Effects to Design Innovative Immunotherapies Targeting Cytokine Activity to Specific Cell Types". Front Immunol. 5: 526. doi:10.3389/fimmu.2014.00526. PMC 4214202
 . PMID 25400632.
. PMID 25400632. - ↑ Thomas C, Moraga I, Levin D, Krutzik PO, Podoplelova Y, Trejo A, Lee C, Yarden G, Vleck SE, Glenn JS, Nolan GP, Piehler J, Schreiber G, Garcia KC (Aug 2011). "Structural linkage between ligand discrimination and receptor activation by type I interferons". Cell. 146 (4): 621–32. doi:10.1016/j.cell.2011.06.048. PMC 3166218
 . PMID 21854986.
. PMID 21854986. - ↑ Platanias LC (May 2005). "Mechanisms of type-I- and type-II-interferon-mediated signalling". Nat Rev Immunol. 5 (5): 375–86. doi:10.1038/nri1604. PMID 15864272.
- ↑ Uzé G, Schreiber G, Piehler J, Pellegrini S (2007). "The receptor of the type I interferon family". Curr Top Microbiol Immunol. 316: 71–95. doi:10.1007/978-3-540-71329-6_5. PMID 17969444.
- ↑ Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, Stark GR (Dec 2007). "Interferons at age 50: past, current and future impact on biomedicine". Nat Rev Drug Discov. 6 (12): 975–90. doi:10.1038/nrd2422. PMID 18049472.
- ↑ Gajewski TF, Corrales L (2015). "New perspectives on type I IFNs in cancer". Cytokine Growth Factor Rev. In press: 175–8. doi:10.1016/j.cytogfr.2015.01.001. PMID 25630967.
- ↑ Gough DJ, Messina NL, Clarke CJ, Johnstone RW, Levy DE (Feb 2012). "Constitutive type I interferon modulates homeostatic balance through tonic signaling". Immunity. 36 (2): 166–74. doi:10.1016/j.immuni.2012.01.011. PMC 3294371
 . PMID 22365663.
. PMID 22365663. - ↑ McNab F, Mayer-Barber K, Sher A, Wack A, O'Garra A (Feb 2015). "Type I interferons in infectious disease". Nat Rev Immunol. 15 (2): 87–103. doi:10.1038/nri3787. PMID 25614319.
- ↑ Trinchieri G (Sep 2010). "Type I interferon: friend or foe?". J Exp Med. 207 (10): 2053–63. doi:10.1084/jem.20101664. PMC 2947062
 . PMID 20837696.
. PMID 20837696. - ↑ Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U, Murphy KM, Schreiber RD (Sep 2011). "Type I interferon is selectively required by dendritic cells for immune rejection of tumors". J Exp Med. 208 (10): 1989–2003. doi:10.1084/jem.20101158. PMC 3182061
 . PMID 21930769.
. PMID 21930769. - ↑ Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF (Sep 2011). "Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells". J Exp Med. 208 (10): 2005–16. doi:10.1084/jem.20101159. PMC 3182064
 . PMID 21930765.
. PMID 21930765. - 1 2 Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, Fu YX, Auh SL (Apr 2011). "The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity". Cancer Res. 71 (7): 2488–96. doi:10.1158/0008-5472.CAN-10-2820. PMC 3070872
 . PMID 21300764.
. PMID 21300764. - 1 2 Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, Teng MW, Smyth MJ (Apr 2011). "Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy". Proc Natl Acad Sci U S A. 108 (17): 7142–7. doi:10.1073/pnas.1016569108. PMC 3084100
 . PMID 21482773.
. PMID 21482773. - 1 2 Schiavoni G, Sistigu A, Valentini M, Mattei F, Sestili P, Spadaro F, Sanchez M, Lorenzi S, D'Urso MT, Belardelli F, Gabriele L, Proietti E, Bracci L (Feb 2011). "Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis". Cancer Res. 71 (3): 768–78. doi:10.1158/0008-5472.CAN-10-2788. PMID 21156650.
- ↑ Lichtman EI, Helfgott SM, Kriegel MA (Jun 2012). "Emerging therapies for systemic lupus erythematosus--focus on targeting interferon-alpha". Clin Immunol. 143 (3): 210–21. doi:10.1016/j.clim.2012.03.005. PMC 3358492
 . PMID 22525889.
. PMID 22525889. - 1 2 Antonelli G, Scagnolari C, Moschella F, Proietti E (2015). "Twenty-five years of type I interferon-based treatment: A critical analysis of its therapeutic use". Cytokine Growth Factor Rev. In press: 121–31. doi:10.1016/j.cytogfr.2014.12.006. PMID 25578520.
- ↑ Sleijfer S, Bannink M, Van Gool AR, Kruit WH, Stoter G (Dec 2005). "Side effects of interferon-alpha therapy". Pharm World Sci. 27 (6): 423–31. doi:10.1007/s11096-005-1319-7. PMID 16341948.
- ↑ Garcin G, Paul F, Staufenbiel M, Bordat Y, Van der Heyden J, Wilmes S, Cartron G, Apparailly F, De Koker S, Piehler J, Tavernier J, Uzé G (2014). "High efficiency cell-specific targeting of cytokine activity". Nat Commun. 5: 3016. doi:10.1038/ncomms4016. PMID 24398568.