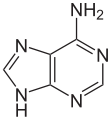
Hypoxanthine
 | |
| Names | |
|---|---|
| IUPAC name
1H-purin-6(9H)-one | |
| Identifiers | |
| 68-94-0 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:17368 |
| ChEMBL | ChEMBL1427 |
| ChemSpider | 768 |
| ECHA InfoCard | 100.000.634 |
| 4555 | |
| KEGG | C00262 |
| MeSH | Hypoxanthine |
| PubChem | 790 |
| UNII | 2TN51YD919 |
| |
| |
| Properties | |
| C5H4N4O | |
| Molar mass | 136.112 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Hypoxanthine is a naturally occurring purine derivative. It is occasionally found as a constituent of nucleic acids, where it is present in the anticodon of tRNA in the form of its nucleoside inosine. It has a tautomer known as 6-hydroxypurine. Hypoxanthine is a necessary additive in certain cell, bacteria, and parasite cultures as a substrate and nitrogen source. For example,[1] it is commonly a required reagent in malaria parasite cultures, since Plasmodium falciparum requires a source of hypoxanthine for nucleic acid synthesis and energy metabolism.
In August 2011, a report, based on NASA studies with meteorites found on Earth, was published suggesting hypoxanthine and related organic molecules, including the DNA and RNA components adenine and guanine, may have been formed extraterrestrially in outer space.[2][3][4]
The Pheretima aspergillum worm, used in Chinese medicine preparations, contains hypoxanthine.[5]
Reactions
It is one of the products of the action of xanthine oxidase on xanthine. However, more frequently in purine degradation, xanthine is formed from reduction of hypoxanthine by xanthine oxidoreductase.
Hypoxanthine-guanine phosphoribosyltransferase converts hypoxanthine into IMP in nucleotide salvage.
Hypoxanthine is also a spontaneous deamination product of adenine. Because of its resemblance to guanine, the spontaneous deamination of adenine can lead to an error in DNA transcription/replication, as it binds with cytosine.
Additional images
References
- ↑ "3H-hypoxanthine uptake inhibition assay for drug susceptibility". WWARN. Retrieved 2012-03-01.
- ↑ Callahan; Smith, K.E.; Cleaves, H.J.; Ruzica, J.; Stern, J.C.; Glavin, D.P.; House, C.H.; Dworkin, J.P. (11 August 2011). "Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases". PNAS. doi:10.1073/pnas.1106493108. Retrieved 2011-08-15.
- ↑ Steigerwald, John (8 August 2011). "NASA Researchers: DNA Building Blocks Can Be Made in Space". NASA. Retrieved 2011-08-10.
- ↑ ScienceDaily Staff (9 August 2011). "DNA Building Blocks Can Be Made in Space, NASA Evidence Suggests". ScienceDaily. Retrieved 2011-08-09.
- ↑ The Pharmacology of Chinese Herbs, Second Edition By Kee C. Huang
External links
- Hypoxanthine at the US National Library of Medicine Medical Subject Headings (MeSH)