Bisulfide
| | |
| Names | |
|---|---|
| Systematic IUPAC name
sulfanide | |
Other names
| |
| Identifiers | |
| 15035-72-0 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:29919 |
| ChEMBL | ChEMBL38703 |
| ChemSpider | 4224877 |
| 24766 | |
| PubChem | 5047209 |
| |
| |
| Properties | |
| HS− | |
| Molar mass | 33.07 g·mol−1 |
| Acidity (pKa) | >14 |
| Basicity (pKb) | <0 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bisulfide (systematically named sulfanide and hydrogen(sulfide)(1−)) is an inorganic anion with the chemical formula HS− (also written as SH−). It contributes no color to bisulfide salts, and its salts may have a distinctive putrid smell. It is classified as a strong base, bisulfide solutions are corrosive and attack the skin.
Bisulfide is the simplest thiolate. It is an important chemical reagent and industrial chemical, mainly used in textiles, synthetic flavors, coloring brasses, and iron control.
Chemical properties
A variety of salts are known, including sodium hydrosulfide and potassium hydrosulfide. Ammonium hydrosulfide, a component of "stink bombs" has not been isolated as a pure solid. Some compounds described as salts of the sulfide dianion contain primarily hydrosulfide. For example, the hydrated form of sodium sulfide, nominally with the formula Na2S · 9 H2O, is better described as NaSH · NaOH · 8 H2O.
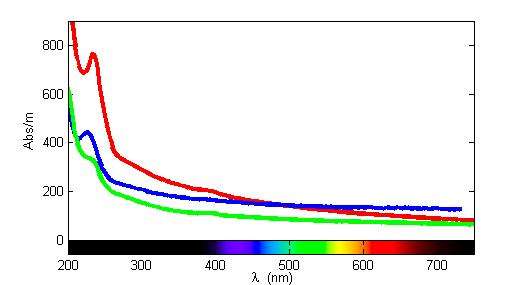
Aqueous bisulfide absorbs light at around 230 nm in the UV/VIS spectrum.[1] Research groups have used field spectrometers to measure the absorption due to bisulfide (and hence its concentration) continuously in the ocean[2][3] and in sewage.[4]
Bisulfide is sometimes confused with the disulfide dianion, S2−
2, or −S–S−.
Basicity
The sulfanidyl segment (–S−) in thiolates such as bisulfide can assimilate a proton by recombination:
- HS− + H+ → H2S
Because of this capture of a proton (H+), bisulfide has basic character. In aqueous solution, it has a primary pKa value of 6.9. Its conjugate acid is hydrogen sulfide (H2S). However, bisulfide's basicity stems from its behavior as an Arrhenius base. A 1.0 M solution containing spectator-only counter ions, has a basic pH, indicating that most of the bisulfide is unassociated.
- HS− + H2O H2S + HO−
Chemical reactions
Bisulfide undergoes the typical chemical reactions of a thiolate. Upon treatment with a standard acid, it converts to hydrogen sulfide and metal salt. With strong acids, it can be doubly protonated to give H
3S+
. Oxidation of bisulfide gives sulfate. When strongly heated, bisulfide salts decompose to produce sulfide salts and hydrogen sulfide.
- 2 HS− → H2S + S2−
Biochemistry
At physiological pH, hydrogen sulfide is usually fully ionized to bisulfide (HS−) so in biochemical settings, "hydrogen sulfide" is often used to mean, bisulfide or hydrosulfide. Hydrosulfide has been identified as the third gasotransmitter along with nitric oxide and carbon monoxide. Its specific role and direct interaction with signaling molecules is the subject of ongoing research.[5]
Other derivatives
SH− is a soft anionic ligand that forms complexes with most metal ions. Examples include [Au(SH)2]− and (C5H5)2Ti(SH)2, derived from gold(I) chloride and titanocene dichloride, respectively.[6]
Safety
Bisulfide salts are corrosive to skin and must, therefore, be handled with appropriate care, since it can cause skin burns, permanent eye damage, and irritation to the mucous membranes. Latex gloves offer no protection, so specially resistant gloves, such as those made of nitrile rubber, are worn when handling its salts. Due to incompatibilities, it is recommended to keep bisulfide salts away from acids, peroxides, zinc, aluminum, copper and its alloys.
See also
References
- ↑ Goldhaber, M.B.; Kaplan, I.R. (1975), "Apparent dissociation constants of hydrogen sulfide in chloride solutions", Marine Chemistry, 3 (1): 83–104, doi:10.1016/0304-4203(75)90016-X
- ↑ Johnson, K.S.; Coletti, L.S. (2001), "In situ ultraviolet spectrophotometery for high resolution and long-term monitoring of nitrate, bromide and bisulfide in the ocean.", Deep-Sea Research, 1 (49): 1291–1305
- ↑ Guenther, E.A.; Johnson, K.S.; Coale, K.H. (2001), "Direct ultraviolet spectrophotometric determination of total sulfide and iodide in natural waters", Analytical Chemistry, 73 (14): 3481–3487, doi:10.1021/ac0013812, PMID 11476251
- ↑ Sutherland-Stacey, L.; Corrie, S.; Neethling, A.; Johnson, I.; Gutierrez, O.; Dexter, R.; Yuan, Z.; Keller, J.; Hamilton, G. (2007), "Continuous measurement of dissolved sulfide in sewer systems", Water Science and Technology
- ↑ J. W. Pavlik, B. C. Noll, A. G. Oliver, C. E. Schulz, W. R. Scheidt, “Hydrosulfide (HS−) Coordination in Iron Porphyrinates”, Inorganic Chemistry, 2010, vol. 49(3), 1017-1026.
- ↑ Peruzzini, M.; de los Rios, I. & Romerosa, A. (2001), "Coordination Chemistry of transition metals with hydrogen chalcogenide and hydrogen chalcogenido ligands", Progress in Inorganic Chemistry, Progress in Inorganic Chemistry, 49: 169–543, doi:10.1002/9780470166512.ch3, ISBN 978-0-470-16651-2.