Hydron (chemistry)
 | |
| Names | |
|---|---|
| Systematic IUPAC name | |
| Identifiers | |
| 12408-02-5 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:15378 |
| ChemSpider | 1010 |
| 2346 | |
| KEGG | C00080 |
| PubChem | [https://pubchem.ncbi.nlm.nih.gov/compound/1038 |
| |
| |
| Properties | |
| H+ | |
| Molar mass | 1.01 g·mol−1 |
| Thermochemistry | |
| Std molar entropy (S |
108.95 J K−1 mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
In chemistry, a hydron is the general name for a cationic form of atomic hydrogen, represented with the symbol H+
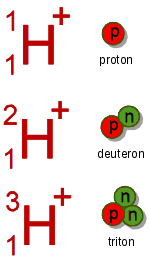
. However, in most textbooks, this term is avoided and instead "proton" is used, which strictly speaking refers to the cation of protium, the most common isotope of hydrogen. The term "hydron" includes cations of hydrogen regardless of their isotopic composition: thus it refers collectively to protons (1H+) for the protium isotope, deuterons (2H+ or D+) for the deuterium isotope, and tritons (3H+ or T+) for the tritium isotope. Unlike other ions, the hydron consists only of a bare atomic nucleus.
The hydron (a completely free or "naked" hydrogen atomic nucleus) is too reactive to occur in many liquids, even though it is sometimes visualized to do so by students of chemistry. A free hydron would react with a molecule of the liquid to form a more complicated cation. Examples are the hydronium ion in water-based acids, and H
2F+
, the unstable cation of fluoroantimonic acid, the strongest superacid. For this reason, in such liquids including liquid acids, hydrons diffuse by contact from one complex cation to another, via the Grotthuss mechanism.[2]
The hydrated form of the hydrogen cation, the hydronium (hydroxonium) ion H
3O+
(aq), is a key object of Arrhenius' definition of acid. Other hydrated forms, the Zundel cation H
5O+
2 which is formed from a proton and two water molecules, and . The hydron itself is crucial in more general Brønsted–Lowry acid–base theory, which extends the concept of acid–base chemistry beyond aqueous solutions.
The negatively charged counterpart of the hydron is the hydride anion, H−
.
Isotopes of hydron

- Proton, having the symbol p or 1H+, is the +1 ion of protium, 1H.
- Deuteron, having the symbol 2H+ or D+, is the +1 ion of deuterium, 2H or D.
- Triton, having the symbol 3H+ or T+, is the +1 ion of tritium, 3H or T.
Other isotopes of hydrogen are too unstable to be relevant in chemistry.
History of the term
The term "hydron" is recommended by IUPAC to be used instead of "proton" if no distinction is made between the isotopes proton, deuteron and triton, all found in naturally occurring undifferentiated isotope mixtures. The name "proton" refers to isotopically pure 1H+.[3] On the other hand, referring to the hydron as simply hydrogen ion is not recommended because hydrogen anions also exist.[4]
The term "hydron" was defined by IUPAC in 1988.[5][6]
Traditionally, the term "proton" was and is used in place of "hydron".
The latter term is generally only used in the context where comparisons between the various isotopes of hydrogen is important (as in the kinetic isotope effect or hydrogen isotopic labeling). Otherwise, referring to hydrons as protons is still considered acceptable, for example in such terms as protonation, deprotonation, proton pump or proton channel. The transfer of H+
in an acid-base reaction is usually referred to as proton transfer. Acid and bases are referred to as proton donors and acceptors correspondingly.
However, although 99.9844% of natural hydrogen nuclei are protons, the remainder (about 156 per million in sea water) are deuterons (see deuterium). A rare triton also occurs (see tritium).
See also
References
- 1 2 "hydron (CHEBI:15378)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute.
- ↑ Computer modeling of proton-hopping in superacids.
- ↑ Nomenclature of Inorganic Chemistry-IUPAC Recommendations 2005 Red Book 2005.pdf IR-3.3.2, p.48
- ↑ Compendium of Chemical Terminology, 2nd edition McNaught, A.D. and Wilkinson, A. Blackwell Science, 1997 [ISBN 0-86542-684-8], also online
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "hydron".
- ↑ Bunnet, J.F.; Jones, R.A.Y. (1968). "Names for hydrogen atoms, ions, and groups, and for reactions involving them (Recommendations 1988)" (PDF). Pure Appl. Chem. 60 (7): 1115–6. doi:10.1351/pac198860071115.