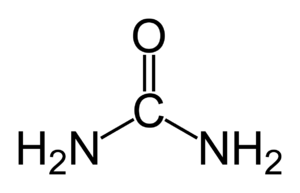
Hydrogen peroxide - urea
 | |
 | |
 | |
| Names | |
|---|---|
| Other names
Urea peroxide, percarbamide, UHP | |
| Identifiers | |
| 124-43-6 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:75178 |
| ChemSpider | 29034 |
| ECHA InfoCard | 100.004.275 |
| PubChem | 31294 |
| UNII | 31PZ2VAU81 |
| |
| |
| Properties | |
| CH6N2O3 | |
| Molar mass | 94.07 g·mol−1 |
| Appearance | White solid |
| Melting point | 75 to 91.5 °C (167.0 to 196.7 °F; 348.1 to 364.6 K) (decomposes) |
| Pharmacology | |
| D02AE01 (WHO) | |
| Hazards | |
| Safety data sheet | External MSDS |
| EU classification (DSD) |
Explosive, |
| Flash point | 60 °C (140 °F; 333 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Hydrogen peroxide - urea is a solid composed of equal amounts of hydrogen peroxide and urea. This compound is a white crystalline solid, which dissolves in water to give free hydrogen peroxide. Often called carbamide peroxide in the dental applications, it is used as a source of hydrogen peroxide for bleaching, disinfection, and oxidation. Hydrogen peroxide - urea contains solid and water-free hydrogen peroxide, which offers a higher stability and better controllability than liquid hydrogen peroxide when used as oxidizing agent.
Production
For the preparation of the compound urea (which is stable to oxidizing agents such as hydrogen peroxide) is dissolved in 30% hydrogen peroxide (molar ratio 2:3) at temperatures below 60 °C. On cooling, hydrogen peroxide - urea precipitates in the form of small platelets.[1]

Determination of the hydrogen peroxide content by titration with potassium permanganate solution gives a value of 35.4% which corresponds to 97.8% of the theoretical maximum value. The remaining impurity consists of urea.
The adduct can be stabilized by adding about 1% sodium pyrophosphate, sodium hexametaphosphate, dihydroxybutanedioic acid or EDTANa2, since these complex the catalytically active heavy metal ions.
Akin to water of crystallization, hydrogen peroxide cocrystallizes with urea with the stoichiometry of 1:1. The compound is simply produced (on a scale of several hundred tonnes a year) by the dissolution of urea in excess concentrated hydrogen peroxide solution, followed by crystallization.[2] The laboratory synthesis is analogous.[3]
Structure and properties
Hydrogen peroxide-urea is a readily water-soluble, odorless, crystalline solid, which is available as white powder or colorless needles or platelets.[1] Upon dissolving in various solvents, the 1:1 complex dissociates back to urea and hydrogen peroxide. So just like hydrogen peroxide, the adduct is an oxidizer but the release at room temperature in the presence of catalysts hydrogen peroxide proceeds in a controlled manner, thus the compound is suitable as a safe substitute for the unstable aqueous solution of hydrogen peroxide. Because of the tendency for thermal decomposition, which accelerates at temperatures above 82 °C,[4] it should not be heated above 60 °C, particularly in pure form.
The solubility of commercial samples varies from 0.05 g/mL[5] to more than 0.6 g/mL.[6] The solid state structure of this adduct at the right has been determined by neutron diffraction.[7]
Applications
Disinfectant and bleaching agent
Hydrogen peroxide - urea is mainly used as a disinfecting and bleaching agent in cosmetics and pharmaceuticals.[2] As a drug, this compound is used in some preparations for the whitening of teeth.[2][8][9] It is also used to relieve minor inflammation of gums, oral mucosal surfaces and lips including canker sores and dental irritation,[10] and to emulsify and disperse ear wax.[11]
Carbamide peroxide is also suitable as a disinfectant, e.g. for germ reduction on contact lens surfaces or as an antiseptic for mouthwashes, ear drops or for superficial wounds and ulcers.
Reagent in organic synthesis
In the laboratory, it is used as a more easily handled replacement for hydrogen peroxide.[3][12][13] It has proven to be a stable, easy-to-handle and effective oxidizing agent which is readily controllable by a suitable choice of the reaction conditions. It delivers oxidation products in a environmentally friendly manner and often in high yields especially in the presence of organic catalysts such as cis-butenedioic anhydride[14] or inorganic catalysts such as sodium tungstate.[15]

It converts thiols selectively to disulfides,[14] secondary alcohols to ketones,[15] sulfides to sulfoxides and sulfones,[16] nitriles to amides,[16][17] N-heterocycles to aminoxides,[16][18]

aromatic hydroxyaldehydes to divalent phenols (Dakin reaction)[16][19] and gives under suitable conditions the corresponding benzoic acids.[19]

It oxidizes ketones to esters, in particular cyclic ketones, such as substituted cyclohexanones[20] or cyclobutanones[21] to give lactones (Baeyer-Villiger oxidation).
The epoxidation of various alkanes in the presence of benzonitrile yields oxiranes in yields of 79 to 96%.[22]

The oxygen atom transferred to the alkene originates from the peroxoimide acid formed intermediately from benzonitrile. The resulting imidic acid tautomerizes to the benzamide.
Safety
The compound acts as a strong oxidizing agent and can cause skin irritation and severe eye damage.[23]
See also
References
- 1 2 C.-S. Lu, E.W. Hughes, P.A. Giguère, "The crystal structure of the urea-hydrogen peroxide addition compound CO(NH2)2 H2O2" (in German), J. Am. Chem. Soc. 63 (6): pp. 1507–1513, doi:10.1021/ja01851a007
- 1 2 3 Harald Jakob, Stefan Leininger, Thomas Lehmann, Sylvia Jacobi, Sven Gutewort (2005), "Peroxo Compounds, Inorganic", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a19_177.pub2
- 1 2 Yu, Lei; Meng, Bo; Huang, Xian (2008). "Urea-Hydrogen Peroxide Complex: A Selective Oxidant in the Synthesis of 2-Phenylselenyl-1,3-butadienes". Synthetic Communications. 38 (18): 3142. doi:10.1080/00397910802109224.
- ↑ H. Heaney, F. Cardona, A. Goti, A.L. Frederick, "Hydrogen Peroxide-Urea", e-EROS Encyclopedia of Reagents for Organic Synthesis, doi:10.1002/047084289X.rh047.pub3
- ↑ Sigma-Aldrich specification sheet
- ↑ Chemicalland data sheet
- ↑ C.J. Fritchie, Jr.; R.K. McMullan; McMullan, R. K. (1981). "Neutron diffraction study of the 1:1 urea:hydrogen peroxide complex at 81 K". Acta Crystallographica Section B. 37 (5): 1086. doi:10.1107/S0567740881005116.
- ↑ Mokhlis, GR; Matis, BA; Cochran, MA; Eckert, GJ (2000). "A Clinical Evaluation of Carbamide Peroxide and Hydrogen Peroxide Whitening Agents during Daytime Use". Journal of the American Dental Association (1939). 131 (9): 1269–77. doi:10.14219/jada.archive.2000.0380. PMID 10986827.
- ↑ Toothwhitening from the UMD of New Jersey website
- ↑ Center for Integrative Medicine: Carbamide Peroxide from the University of Maryland Medical Center website Archived October 18, 2007, at the Wayback Machine.
- ↑ "Carbamide peroxide (Into the ear)". Umm.edu. University of Maryland Medical Center. Retrieved July 29, 2015.
- ↑ Varma, Rajender S.; Naicker, Kannan P. (1999). "The Urea−Hydrogen Peroxide Complex: Solid-State Oxidative Protocols for Hydroxylated Aldehydes and Ketones (Dakin Reaction), Nitriles, Sulfides, and Nitrogen Heterocycles". Organic Letters. 1 (2): 189. doi:10.1021/ol990522n.
- ↑ Harry Heaney, Francesca Cardona, Andrea Goti, "Hydrogen Peroxide–Urea" Encyclopedia of Reagents for Organic Synthesis 2008. doi:10.1002/047084289X.rh047.pub2
- 1 2 B. Karami, M. Montazerozohori, M. H. Habibi, "Urea-Hydrogen Peroxide (UHP) oxidation of thiols to the corresponding disulfides promoted by maleic anhydride as mediator" (in German), molecules 10 (10): pp. 1358–1363, doi:10.3390/10101385, http://www.mdpi.org/molecules/papers/10101358.pdf
- 1 2 M. Lukasiewicz, D. Bogdal, J. Pielichowski. "Microwave-assisted oxidation of alcohols using urea hydrogen peroxide". 8th International Electronic Conference on Synthetic Organic Chemistry. ECSOC-8. Retrieved 2016-05-10.
- 1 2 3 4 R.S. Varma, K.P. Naicker, "The Urea-Hydrogen Peroxide Complex: Solid-State Oxidative Protocols for Hydroxylated Aldehydes and Ketones (Dakin Reaction), Nitriles, Sulfides, and Nitrogen Heterocycles" (in German), Org. Lett. 1 (2): pp. 189–191, doi:10.1021/ol990522n
- ↑ US 0
- ↑ D. Rong, V.A. Phillips, R.S. Rubio, M.A. Castro, R.T. Wheelhouse, "A safe, convenient and efficient method for the preparation of heterocyclic N-oxides using urea-hydrogen peroxide" (in German), Tetrahedron Lett. 49 (48): pp. 6933–6935, doi:10.1016/tetlet.2008.09.124
- 1 2 H. Heaney, A.J. Newbold, "The oxidation of aromatic aldehydes by magnesium monoperoxyphthalate and urea-hydrogen peroxide" (in German), Tetrahedron Lett. 42 (37): pp. 6607–6609, doi:10.1016/S0040-4039(01)01332-6
- ↑ M.Y. Rios, E. Salazar, H.F. Olivo, "Baeyer–Villiger oxidation of substituted cyclohexanones via lipase-mediated perhydrolysis utilizing urea–hydrogen peroxide in ethyl acetate" (in German), Green Chem. 9: pp. 459–462, doi:10.1039/B618175A
- ↑ A. Watanabe, T. Uchida, K. Ito, T. Katsuki, "Highly enantioselective Baeyer-Villiger oxidation using Zr(salen) complex as catalyst" (in German), Tetrahedron Lett. 43 (25): pp. 4481–4485, doi:10.1016/S0040-4039(02)00831-6
- ↑ L. Ji, Y.-N. Wang, C. Qian, X.-Z. Chen, "Nitrile-promoted alkene epoxidation with urea-hydrogen peroxide (UHP)" (in German), Synth. Commun. 43 (16): pp. 2256–2264, doi:10.1080/00397911.2012.699578
- ↑
External links
- "Hydrogen peroxide urea adduct, UHP". Organic Chemistry Portal.
- "Carbamide Peroxide Monograph". Drugs.com.