Hormone replacement therapy (menopause)
| Menopause | |
|---|---|
| Classification and external resources | |
| Specialty | urology |
| ICD-10 | N95.0 |
| ICD-9-CM | 627.2 |
| DiseasesDB | 8034 |
| MedlinePlus | 000894 |
| eMedicine | article/264088 |
Hormone replacement therapy (HRT) in menopause is medical treatment in surgically menopausal, perimenopausal and postmenopausal women. Its goal is to mitigate discomfort caused by diminished circulating estrogen and progesterone hormones in menopause. Combination HRT is often recommended as it decreases the amount of endometrial hyperplasia and cancer associated with unopposed estrogen therapy.[1][2] The main hormones involved are estrogen, progesterone and progestin. Some recent therapies include the use of androgens as well.[3]
The 2002 Women's Health Initiative of the National Institutes of Health found disparate results for all cause mortality with hormone replacement, finding it to be lower when HRT was begun earlier, between age 50-59, but higher when begun after age 60. In older patients, there was an increased incidence of breast cancer, heart attacks and stroke, although a reduced incidence of colorectal cancer and bone fracture.[4] Some of the WHI findings were again found in a larger national study done in the UK, known as The Million Women Study. As a result of these findings, the number of women taking hormone treatment dropped precipitously.[5] The Women's Health Initiative recommended that women with non-surgical menopause take the lowest feasible dose of HRT for the shortest possible time to minimize associated risks.[4]
The current indications for use from the U.S. Food and Drug Administration include short-term treatment of menopausal symptoms, such as vasomotor hot flashes or urogenital atrophy, and prevention of osteoporosis.[6] In 2012, the United States Preventive Task Force concluded that the harmful effects of combined estrogen and progestin are likely to exceed the chronic disease prevention benefits in most women.[7][8] A consensus expert opinion published by The Endocrine Society stated that when taken during perimenopause, or the initial years of menopause, hormonal therapy carries significantly fewer risks than previously published, and reduces all cause mortality in most patient scenarios.[9] The American Association of Clinical Endocrinology also released a position statement in 2009 that approved of HRT in appropriate clinical scenarios.
Health effects
There have been a number of large scale cross sectional and cohort studies on the effects of hormone replacement in menopause, the largest being in the United States, the United Kingdom and China. Demographically, the vast majority of data available is in post-menopausal American women with concurrent pre-existing conditions, and with a mean age of over 60 years.[10]
The North American Menopause Society (NAMS) 2016 annual meeting mentioned that hormone therapy may have more benefits than risks when it comes to females under the age of 60 years old.[11]
In 2002 the Women's Health Initiative (WHI) was published. That study looked at the effects of hormonal replacement therapy in post-menopausal women. Both age groups had a slightly higher incidence of breast cancer, and both heart attack and stroke were increased in older patients, although not in younger participants. In fact, the use of hormone therapy in the United States has actually dropped greatly since 2002.[12] Progesterone is the major anabolic hormone for breast tissue, and accordingly breast cancer was not increased in patients who were on estrogen therapy alone after hysterectomy. Treatment with unopposed estrogen (the supplementation of endogenous estrogens without a progestogen) is contraindicated if the uterus is still present, due its proliferative effect on the endometrium. The WHI also found a reduced incidence of colorectal cancer when estrogen and progesterone were used together, and most importantly, a reduced incidence of bone fractures. Ultimately, the study found disparate results for all cause mortality with hormone replacement, finding it to be lower when HRT was begun during ages 50–59, but higher when begun after age 60.[4] Some findings of the WHI were reconfirmed in a larger national study done in the UK, known as The Million Women Study. Coverage of the WHI findings led to a reduction in the number of post-menopausal women on hormone replacement therapy.[13] The authors of the study recommended that women with non-surgical menopause take the lowest feasible dose of HRT, and for the shortest possible time, to minimize risk.[4]
The data published by the WHI suggested supplemental estrogen increased risk of venous emboli and breast cancer but was protective against osteoporosis and colorectal cancer, while the impact on cardiovascular disease was mixed.[14] These results were later confirmed in trials from the United Kingdom, but not in more recent studies from France and China. Genetic polymorphism appears to be associated with inter-individual variability in metabolic response to HRT in postmenopausal women.[15][16]
These recommendations have not held up with further data analysis, however. Subsequent findings released by the WHI showed that all cause mortality was not dramatically different between the groups receiving conjugated equine estrogen (CEE), those receiving estrogen and progesterone, and those not on HRT at all. Specifically, the relative risk for all-cause mortality was 1.04 (confidence interval 0.88–1.22) in the CEE-alone trial and 1.00 (CI, 0.83–1.19) in the estrogen plus progesterone trial.[17] Further, in analysis pooling data from both trials, post menopausal HRT was associated with a significant reduction in mortality (RR, 0.70; CI, 0.51–0.96) among women ages 50 to 59. This would represent five fewer deaths per 1,000 women per 5 years of therapy.
However, neither the WHI nor the Million Women Study differentiated the results for different types of synthetic progesterones used. Medroxyprogesterone acetate (MPA) -- the type most commonly used in the United States—was the only one examined by the WHI, which in its analysis and conclusions extrapolated the benefits versus risks of MPA to all synthetic progesterones. This conclusion has since been challenged by several researchers as unjustified and misleading, resulting in unreasonable, unnecessary avoidance by many women of HRT for menopause. In fact, primate research indicates that the side effects of MPA may be much worse than those of other synthetic progesterones, and some human studies indicate that MPA may be responsible for negating the protective cardiac benefits of estrogen that were found for estrogen-only HRT users. Critics including Bethea note that there are now research papers showing significantly better outcomes in brain, breast, and cardiovascular parameters with estradiol plus progesterone instead of MPA and conclude that further studies are needed to know more precisely what the differences in effects are when other synthetic progesterones are used versus natural progesterone in HRT for menopause, so that women aren't needlessly discouraged from seeking HRT for menopause.[18][19][20][21]
A robust Bayesian meta-analysis from 19 randomized clinical trials reported similar data with a RR of mortality of 0.73 (CI, 0.52–0.96) in women younger than age 60.[22] However, MHT had minimal effect among those between 60 and 69 years of age (RR, 1.05; CI, 0.87–1.26) and was associated with a borderline significant increase in mortality in those between 70 and 79 years of age (RR, 1.14; CI, 0.94 –1.37; P for trend < 0.06).[23] Similarly, in the HERS trial, with participants having a mean age of 66.7 yr, MHT did not reduce in total mortality (RR, 1.08; CI, 0.84 –1.38).[24] A 2003 meta-analysis of 30 randomized trials of menopausal HRT in relation to mortality showed that it was associated with a nearly 40% reduction in mortality in trials in which participants had a mean age of less than 60 yr or were within 10 yr of menopause onset but was unrelated to mortality in the other trials.[25] The findings in the younger age groups were similar to those in the observational Nurses' Health Study (RR for mortality, 0.63; CI, 0.56 – 0.70).[9][26]
The beneficial potential of HRT was bolstered in a consensus expert opinion published by The Endocrine Society, which stated that when taken during perimenopause or the initial years of menopause, hormonal therapy carries significantly fewer risks than previously published and reduces all cause mortality in most patient scenarios.[9] The American Association of Clinical Endocrinology released a position statement in 2009 that approved of HRT in the appropriate clinical scenario.
Proprietary mixtures of progestins and conjugated equine estrogens are a commonly prescribed form of HRT. As the most common and longest-prescribed type of estrogen used in HRT, most studies of HRT involve CEE. More recently developed forms of drug delivery include suppositories, subdermal implants, skin patches and gels. They have more local effect, lower doses, fewer side effects, and result in constant rather than cyclical serum hormone levels.[27]
Management of sexual dysfunction
Hormone replacement therapy's goal is to mitigate discomfort caused by diminished circulating estrogen and progesterone hormones in menopause. In those with premature or surgically induced menopause, a combination HRT is often recommended, as it may also prolong life and may decrease a woman's chances of developing endometrial cancers associated with unopposed estrogen therapy, as well by decreasing the incidence of dementia.[1][2] The main hormones involved are estrogen, progesterone and progestin. Some recent therapies include the use of androgens as well.[3]
Data from numerous studies have consistently found that HRT leads to improvements in aspects of post-menopausal sexual dysfunction.[3] Sexuality is a critical aspect of quality of life for the large majority of menopausal women; therefore, any features of the menopausal transition that can negatively affect a woman’s sexuality have the ability to significantly alter her quality of life. The most prevalent of female sexual dysfunctions linked to menopause include lack of desire and low libido, both of which can be explained by changes in hormonal physiology.[2]
Improvements in sexual pain, vaginal lubrication and orgasm are found to be statistically different from those using HRT.[2] Estrogens have positive effects of mood, sexual function, target end organs, and cognitive function. It has also been shown to prevent amyloid plaque formation, oxiadative stress, or deterioration of the cholinergic neurotransmitter system, all of which contribute to the etiology of Alzheimer’s Disease.[28] There are several options of HRTs for women. These can include the use of estrogens alone (ERT), a combination of estrogens with one of several progestins as HRT, or the combination of estrogens, progestins, and androgens as HRT.[28]
Venous and arterial coagulation

Comparisons between orally administered pill and transdermal patch suggests that when estrogens are taken orally the risks of thrombophlebitis and pulmonary embolism are increased, an effect which is not seen with topical administration. Transdermal and transvaginal administration are not subject to first pass metabolism, and so lack the anabolic effects that oral therapy has on hepatic synthesis of Vitamin K dependent clotting factors.[29] This effect refers only to patches for post menopausal hormone replacement, which contain 17 beta estradiol, not those used in oral contraceptive therapy, which contain ethinyl estradiol. The latter is associated with an increased incidence of venous clot.[30] The WHI also showed an increased incidence arterial disease, namely stroke, in patients who began HRT after the age of 65, although this effect was not significantly present in those who began therapy during their fifth decade.
Cardiovascular effects
The impact of hormone replacement on cardiovascular morbidity is a subject of much controversy in the medical literature. The reduced risk of cardiovascular diseases associated with hormone replacement therapy (HRT), reported in observational studies, has not been subsequently confirmed in randomized clinical trials. The increased risk of cardiovascular disease in the WHI was not statistically significant, and only found in the oldest women, and those who started HRT late after menopause began.[31] The increase in risks of coronary heart disease in the treatment arm of the study varied according to age and years since onset of menopause. Women aged 50 to 59 using HRT showed a trend towards lower risk of coronary heart disease,[32] as did women who were within five years of the onset of menopause.[33]
A Cochrane review came to the result that in women starting hormone replacement therapy less than 10 years after menopause have a lower mortality and lower rate of coronary heart disease compared to placebo or no treatment, without any strong evidence of an effect on the risk of stroke. Those starting therapy more than 10 years after menopause have little effect on mortality and coronary heart disease, but have an increased risk of stroke. Overall, however, taking the increased risk of venous thromboembolism into account, it came to the conclusion that has hormone replacement therapy has little if any benefit for primary or secondary prevention of cardiovascular disease.[34]
The adverse cardiovascular outcomes may only apply to oral dosing with the progestin and equine estrogens in oral systemic therapy, while topical estradiol and estriol may not produce the same risks, due to the absence of anabolic effects of hepatic vitamin K dependent clotting factors.[30]
On a molecular level, hormone replacement therapy at the time of menopause has effects on the lipid profile. Specifically, HDL decreases, while LDL, triglycerides and lipoprotein a increase. Supplemental estrogen improves the lipid profile by reversing each of these effects. Beyond this, it improves cardiac contractility, coronary artery blood flow, metabolism of carbohydrates, and decreases platelet aggregation and plaque formation. At the molecular level HRT may promote reverse cholesterol transport (RCT) via the induction of cholesterol ABC transporters.[35]
Endometrial effects

While combined estrogen-progesterone supplementation has been linked to an increased incidence of endometrial cancer, the specific subtype is usually stage I, or in situ, and has extremely low morbidity and mortality, and studies in American women have shown the tumor to not have propensity for growth into the myometrium or parametrial soft tissues. When seen in the context of all cause mortality, women who take estrogen and develop endometrial cancer have higher survival rates than women who do not take hormonal therapy at all, which was due to the preventive effect of HRT on hip fractures.
Unopposed estrogen can also result in endometrial hyperplasia, a precursor to endometrial cancer. The extensive use of high-dose estrogens for birth control in the 1970s is thought to have resulted in a significant increase in the incidence of this type of cancer.[36]
Musculoskeletal effects
HRT is effective at reversing the effects of aging on muscle.[37]
Neurologic effects
According to a 2007 presentation at an American Academy of Neurology meeting,[38] hormone therapy taken soon after menopause may help protect against dementia, but it raises the risk of mental decline in women who do not take hormone replacement until they are older. Dementia risk was 1% in women who started HRT early, and 1.7% in women who didn't, (i.e. women who didn't take hormone replacement seem to have had—on average—a 70% higher relative risk of dementia than women who began HRT around the time of the beginning of menopause). This suggests that there may be a "critical period" during which time taking HRT may have benefits, but if HRT is initiated after that period, it will not have such benefits and may cause harm. This is consistent with research that hormone therapy improves executive and attention processes in postmenopausal women.[39] It is also supported by research upon monkeys that were given ovariectomies to imitate the effect of menopause and then estrogen therapies. This showed replacement treated compared to nontreated monkeys had long term improved prefrontal cortex executive abilities on the Wisconsin Card Sorting Test.[40]
Breast tissue effects
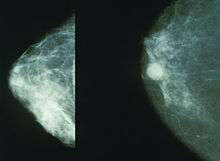
The relative risk (RR) of breast cancer varies from 1.24 in the WHI study to 1.66 in the Million Women Study, with results differing according to interval between menopause and hormone therapy and methods of hormone replacement.[41] The WHI preliminary results in 2004 found a non-significant trend in the estrogen-alone clinical trial towards a reduced risk of breast cancer[32] and a 2006 update concluded that use of estrogen-only HRT for 7 years does not increase the risk of breast cancer in postmenopausal women who have had a hysterectomy.[42] The results of the WHI estrogen-alone trial suggest that the progestin used in the WHI estrogen-plus-progestin trial increased the risk for breast cancer above that associated with estrogen alone.[43] HRT has been more strongly associated with risk of breast cancer in women with low or normal body mass index (BMI) of less than 25, but no association has been observed in women with a high body mass index.[44]
A recent randomized controlled trial recently showed that increased breast cancer risk applied only to those women who take progesterone analogues, but not to those taking bio-identical progesterone itself, nor to hysterectomized women who take unopposed estrogen.[45]
Some reports have not found an association of progesterone therapy and breast cancer. The absence of effect in these studies has been suggested to be due to selective prescription to overweight women, or to the very low progesterone serum levels after oral administration leading to a strong tumor inactivation rate.[46]
For women who previously have had breast cancer, it is recommended to first consider non-hormonal options for menopausal effects, such as bisphosphonates or selective estrogen receptor modulators (SERMs) for steoporosis, cholesterol lowering agents and aspirin for cardiovascular disease and vaginal estrogen for local symptoms. Observational studies of systemic hormone replacement after breast cancer are generally reassuring. If hormone replacement is necessary after breast cancer, estrogen only therapy or estrogen therapy with an intrauterine device with progestogen may be safer options than combined systemic therapy.[47]
Hip fractures
Estrogen prevents the activity of osteoclasts, and improves bone mineral density. Hip fracture is a leading cause of morbidity and mortality in older females, and usually does not occur in the setting of osteoporosis. Estrogen is the only medical therapy that has been shown to prevent hip fractures in women that are not osteoporotic, with efficacy superior to bisphosphonates or calcium and vitamin D supplementation.
Ovarian cancer
A 2015 meta-analysis found that hormone replacement therapy was associated with an increased risk of ovarian cancer. The authors concluded that if this association is causal, women using HRT have about one additional case of ovarian cancer per 1,000 users.[48]
Epigenetic aging effects
HRT appears to slow down the biological/epigenetic aging rate of buccal cells but not that of blood cells .[49] Conversely, the unmitigated loss of hormones resulting from menopause accelerates the biological aging rate of blood [49] according to a molecular biomarker of aging known as epigenetic clock.[50]
Premature stoppage of Women's Health Initiative
Clinical medical practice changed based upon two parallel WHI studies of postmenopausal HRT. Prior studies were smaller, and many were of women who electively took hormonal therapy. The WHI studies were the first large, double-blind, placebo-controlled clinical trials of HRT in healthy, postmenopausal women.
One portion of the parallel studies followed over 16,000 women for an average of 5.2 years, half of whom took placebo, while the other half took a combination of the progestin medroxyprogesterone acetate (Provera) and conjugated equine estrogen (Premarin). The combination of hormones is referred to as Prempro.
This WHI estrogen-plus-progestin trial was stopped prematurely in 2002 because preliminary results suggested risks of combined conjugated equine estrogen and progestin exceeded their benefits. The first report on the halted WHI estrogen-plus-progestin study came out in July 2002.[4]
The study reported statistically significant increases in rates of breast cancer, coronary heart disease, strokes and pulmonary emboli. The study also found statistically significant decreases in rates of hip fracture and colorectal cancer. "A year after the study was stopped in 2002, an article was published indicating that estrogen plus progestin also increases the risks of dementia."[51] The conclusion of the study was that the HRT combination presented risks that outweighed its measured benefits. The results were almost universally reported as risks and problems associated with HRT in general, rather than with Prempro, the specific proprietary combination of conjugated equine estrogen and progestin studied.
After the increased clotting found in the first WHI results was reported in 2002, the number of Prempro prescriptions filled reduced by almost half. Following the WHI results, a large percentage of hormone replacement users opted out of them, which was quickly followed by a sharp drop in breast cancer rates. The decrease in breast cancer rates has continued in subsequent years.[52] An unknown number of women started taking alternatives to Prempro, such as bioidentical hormones, though scientists have asserted that such hormones are not significantly different from Prempro.[53]
The other portion of the parallel studies featured women who were post hysterectomy so who consequently did not need to take a progestin when using estrogen. They were given either placebo or conjugated equine estrogen alone. This group did not show the risks demonstrated in the combination hormone study, and the estrogen-only study was not halted in 2002. However, in February 2004 it, too, was halted. While there was a 23% decreased incidence of breast cancer in the estrogen-only study participants, risks of stroke and pulmonary embolism were increased slightly, predominantly in patients who began HRT over the age of 60.[54]
Women's Health Initiative limitations and criticisms
The WHI trial was limited by low adherence, high attrition, inadequate power to detect risks for some outcomes, and evaluation of few regimens.[8] The double blinding limited validity of study results due to its effects on patient exclusion criteria. Patients who were experiencing symptoms of the menopausal transition were excluded from the study, meaning that younger women who had only recently experienced menopause were not significantly represented. As a result, while the average age of menopause is age 51, study participants were on average 62 years of age. Demographically, the vast majority were Caucasian, and tended to be slightly overweight and former smokers.
Bioidentical hormone replacement therapy
Bioidentical hormone replacement therapy is the use of hormones that are chemically identical to those produced in a woman's body. Proponents also claim that BHRT can offer advantages beyond those typical of traditional HRT. There is no evidence in the peer-reviewed literature that validates or refutes these claims.
Some bioidentical hormone therapies are approved by the United States Food and Drug Administration (FDA). Others are provided in connection with the practices of pharmaceutical compounding and saliva testing to determine, and adjust, a woman's hormone levels. The latter two practices are not widely accepted in clinical medicine. Compounding has not demonstrated any benefits and presents risks of uncertain dosing, potency and possible contamination. In addition, saliva testing is of limited utility due to natural fluctuations in hormone levels, and lack of consensus for ideal dosage in humans.[55]
The FDA has stated that BHRT is unsupported by medical evidence, and its administration is considered false and misleading by the agency. The FDA has expressed concern that unfounded claims like these mislead women and health care professionals. Traditional therapy has been researched to quantify these risks and benefits, and are produced by manufacturers with stringent purity and potency standards.[56]
HRT and sexuality
Menopause is the permanent cessation of menstruation resulting from loss of ovarian follicular activity.[2] Menopause can be divided into early and late transition periods, also known as perimenopause and postmenopause. Each stage is marked by changes in hormonal patterns, which can induce menopausal symptoms.[2] It is possible to induce menopause prematurely by surgically removing the ovary or ovaries (oophorectomy). This is often done as a consequence of ovarian failure, such as ovarian or uterine cancers. The most common side effects of the menopausal transition are: lack of sexual desire or libido, lack of sexual arousal, and vaginal dryness.[2] The modification of women’s physiology can lead to changes in her sexual response, the development of sexual dysfunctions, and changes in her levels of sexual desire.[57]
It is commonly perceived that once women near the end of their reproductive years and enter menopause that this equates to the end of her sexual life.[2] However, especially since women today are living one third or more of their lives in a postmenopausal state, maintaining, if not improving, their quality of life, of which their sexuality can be a key determinant, is of importance.[28] A recent study of sexual activities among women aged 40–69 revealed that 75% of women are sexually active at this age; this indicates that the sexual health and satisfaction of menopausal women are an aspect of sexual health and quality of life that is worthy of attention by health care professionals.[2]
A major complaint among postmenopausal women is decreased libido, and many may seek medical consultation for this.[3] Several hormonal changes take place during the menopausal period, including a decrease in estrogen levels and an increase in follicle-stimulating hormone. For most women, the majority of change occurs during the late perimenopausal and postmenopausal stages.[2] Decrease in other hormones such as the sex hormone-binding globulim (SHBG) and inhibin (A and B) also take place in the postmenopausal period. Testosterone, a hormone more commonly associated with males, is also present in women. It peaks at age 30, but declines with age, so there is little variation across the lifetime and during the menopausal transition.[2] However, in surgically induced menopause, instead of the levels of estrogens and testosterone slowly declining over time, they decline very sharply, resulting in more severe symptoms.[2]
In menopausal women, sexual functioning can impact several dimensions of a woman’s life, including her physical, psychological, and mental well-being.[58] During the onset of menopause, sexuality can be a critical issue in determining whether one begins to experience changes in their sexual response cycle.[59] Both age - and menopause-related events can affect the integrity of a woman’s biological systems involved in the sexual response cycle, which include hormone environment, neuro-muscular substrates, and vascular supplies.[59] Therefore, it can be appropriate to make use of HRT, especially in women with low or declining quality of life due to sexual difficulties.[3]
Current research that has examined the impact of menopause on women’s self-reported sexual satisfaction indicates that 50.3% of women experience some sexual disturbance in one of five domains, and 33.7% experience disturbances in two of the domains.[58] Of these were desire, orgasm, lubrication, and arousal disturbances. With regards to arousal, they found a significant negative association between age and arousal, in that as women aged they were more likely to report lower arousal scores. In the desire and orgasm domains, 38% of women reported a disturbances in their desire, and 17% reported a disturbance in their orgasm capabilities; of the 17%, 14% were premenopausal, 15.2% were postmenopausal and taking a form of HRT, and 22% were postmenopausal not on a form of HRT.[58] Eight percent of women reported disturbances lubricating during sexual activity; 14.3% in the premenopausal group, 30% in the postmenopausal group not using a HRT, and 11.7% among those postmenopausal women using HRT.[58] Lastly, 21% of women reported pain as a disturbance in their sexual satisfaction - the premenopausal group at 14.3%, the postmenopausal women using HRT at 13.3% and the group with the highest rates, similarly to the other results, was the postmenopausal women not taking HRT at 34%.[58] This study concluded that there was a significant decline in sexual function related to menopause in the pain and lubrication domains.[58]
The maintenance and improvement of quality of life during the menopausal period is at the core of estrogen and progestin-based hormone therapy.[3] Both HRT and estrogen replacement therapy (ERT) have been shown to enhance sexual desire in a significant percent of women; however, as with all pharmacological treatments, not all women have been responsive, especially those with preexisting sexual difficulties.[57] ERT restores vaginal cells, pH levels, and blood flow to the vagina, all of which deterioration are associated with the onset of menopause. Dyspareunia (due to vaginal dryness) appears to be the most responsive component of menopausal women’s sexuality to ERT.[57] It also has been shown to have positive effects on the urinary tract and atrophy and may initially improve libido or sexual sensitivity.[57] Other improvements in areas such as sexual desire, arousal, fantasies, and frequency of coitus and orgasm have also been noted.[57] However, the effectiveness of ERT has been shown to decline in some women after long-term use.[57] A number of studies have found that the combined effects of estrogen/androgen replacement therapy can increase a woman’s motivational aspects of sexual behaviour over and above what can be achieved with estrogen therapy alone.[57] Findings on a relatively new form of HRT called tibolone - a synthetic steroid with estrogenic, androgenic, and progestogenic properties - suggest that it has the ability to improve mood, libido, and somatic symptoms of surgically menopausal women to a greater degree than ERT. In various placebo-controlled studies, improvements in vasomotor symptoms, emotional reactions, sleep disturbances, somatic symptoms, and sexual desire have been observed.[3] However, while this is and has been available in Europe for almost two decades, this has not been approved for use in North America at this point.[3]
Effects on sexuality in transgender individuals
Hormone replacement therapy is an integral component in the medical treatment of transgender individuals, as the hormones can lead to decreasing the dichotomy between an individual's body and their gender identity.[60] Managing long-term hormonal regimens have not been studied and are difficult to estimate because research on the long-term use of hormonal therapy has not been noted.[60] However, it is possible to speculate the outcomes of these therapies on transgender people based on the knowledge of the current effects of gonadal hormones on sexual functioning in cisgender men and women.[61]
Firstly, if one is to decrease testosterone in male-to-female gender transition, it is likely that sexual desire and arousal would be inhibited; alternatively, if high doses of estrogen negatively impact sexual desire, which has been found in some research with cisgender women, it is hypothesized that combining androgens with high levels of estrogen would intensify this outcome.[61] Unfortunately, to date there haven’t been any randomized clinical trials looking at the relationship between type and dose of hormone replacement therapy, so the relationship between them remains unclear.[61] Typically, the estrogens given for male-to-female gender transition are 2-3 times higher than the recommended dose for HRT in postmenopausal women.[60] Pharmokinetic studies indicate taking these increased doses may lead to a higher boost in plasma estradiol levels; however, the long-term side effects haven’t been studied and the safety of this route is unclear.[60]
As with any pharmacological or hormone treatment, there are potential side effects, which in the case of hormone therapy include changes in sexual functioning. These have the ability to significantly impact sexual functioning, either directly or indirectly through the various side effects, such as cerebrovascular disorders, obesity, and mood fluctuations.[61] In addition, some research has found an onset of diabetes following feminizing hormone therapy, which impairs sexual response. Whatever route an individual and his or her doctor choose to take, it is important to consider both the medical risks of hormone therapy as well as the psychological needs of the patient.
Contraindications
Absolute contraindications
- Undiagnosed vaginal bleeding
- Severe liver disease
- Pregnancy
- Coronary artery disease (CAD)
- Well-differentiated and early endometrial cancer (once treatment for the malignancy is complete, is no longer an absolute contraindication). Progestins alone may relieve symptoms if the patient is unable to tolerate estrogens.
- Recent DVT or stroke
Relative contraindications
- Migraine headaches
- Personal history of breast cancer
- Personal history of ovarian cancer
- Venous thrombosis
- History of uterine fibroids
- Atypical ductal hyperplasia of the breast
- Active gallbladder disease (cholangitis, cholecystitis)
Adverse effects
|
|
History
The extraction of conjugated estrogens from the urine of pregnant mares led to the marketing in 1943 of the first HRT treatment, Premarin.[63] From that time until 1975, estrogen was administered without supplemental progesterone or progestin. A study from Kaiser Permanente by Dr. Harry Ziel demonstrated that in the absence of progesterone, patients were at increased risk of endometrial cancer with unopposed estrogen therapy. After this, progestin was supplemented in women who had not received surgical hysterectomy, to reduce the incidence of endometrial hyperplasia and cancer. This was followed by the Women's Health Initiative (WHI) in 2002. However, the arm of the WHI receiving combined Estrogen and Progesterone therapy was closed prematurely by its Data Monitoring Committee (DMC) due to perceived health risks, although the trial arm was stopped only a full year after the data suggesting increased risk became manifest. In 2004, the arm of the WHI in which post-hysterectomy patients were being treated with estrogen alone was also closed by the DMC.
Administration and formulations
HRT is available in various forms. It generally provides low dosages of one or more estrogens, and often also provides either progesterone or a chemical analogue, called a progestin. Testosterone may also be included. Some HRT treatments such as Livial contain Tibolone, an anabolic steroid, that has the properties of oestrogen, progestogen and testosterone. Such treatments aren't usually recommended to women who are perimenopausal or for at least 12 months after the last menstrual period.[64]
In women who have had a hysterectomy, an estrogen is usually given without any progesterone, a therapy referred to as "unopposed estrogen therapy". HRT may be delivered to the body via patches, tablets, creams, troches, IUDs, vaginal rings, gels or, more rarely, by injection. For example, vaginally administered estrogens include those given by intravaginal tablets, creams and rings, and can have more effect on atrophic vaginitis with fewer systemic effects than estrogens delivered by other means.[65]
Dosage is often varied cyclically to more closely mimic the ovarian hormone cycle, with estrogens taken daily and progesterone or progestins taken for about two weeks every month or two; a method called "cyclic HRT" or "sequentially combined HRT" (abbreviated scHRT). An alternate method, a constant dosage with both types of hormones taken daily, is called "continuous combined HRT" or ccHRT, and is a more recent innovation. Sometimes an androgen, generally testosterone, is added to treat diminished libido. It may also treat reduced energy and help reduce osteoporosis after menopause.
HRT is often given as a short-term relief (often one or two years, usually less than five) from menopausal symptoms (hot flushes, irregular menstruation, fat redistribution, etc.). Younger women with premature ovarian failure or surgical menopause may use hormone replacement therapy for many years, until the age that natural menopause would be expected to occur.
Formulations of estradiol include:
- Oral tablets, commonly given in amounts of 1 to 2 milligrams of estradiol per day, with trade names including Progynon.
- Estrogen patches
- Estrogen transdermal gels
In addition, there are many formulations of estradiol combined with one of various progestins, such as norethisterone (in Activelle, Novofem and Cliovelle), levonorgestrel, medroxyprogesterone (in Indivina), dienogest or drospirenone.
See also
References
- 1 2 Shuster, Lynne T.; Rhodes, Deborah J.; Gostout, Bobbie S.; Grossardt, Brandon R.; Rocca, Walter A. (2010). "Premature menopause or early menopause: Long-term health consequences". Maturitas. 65 (2): 161–166. doi:10.1016/j.maturitas.2009.08.003. ISSN 0378-5122. PMC 2815011
 . PMID 19733988.
. PMID 19733988. - 1 2 3 4 5 6 7 8 9 10 11 12 Eden, K.J., & Wylie, K.R. (2009). Quality of sexual life and menopause. Women’s Health, 5 (4), 385-396. doi:10.2217/whe.09.24
- 1 2 3 4 5 6 7 8 Ziaei, S., Moghasemi, M., & Faghihzadeh, S. (2010). Comparative effects of conventional hormone replacement therapy and tibolone on climacteric symptoms and sexual dysfunction in postmenopausal women. Climateric, 13, 147-156. doi:10.1016/j.maturitas.2006.04.014
- 1 2 3 4 5 Writing Group for the Women's Health Initiative Investigators (2002). "Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results From the Women's Health Initiative Randomized Controlled Trial". JAMA. 288 (3): 321–333. doi:10.1001/jama.288.3.321. PMID 12117397.
- ↑ Chlebowski, RT; Kuller LH; et al. (2009). "Breast cancer after use of estrogen plus progestin in postmenopausal women". NEJM. 360 (6): 573–87. doi:10.1056/NEJMoa0807684. PMID 19196674.
- ↑ "USPTF Consensus Statement". 2012.
- ↑ Kreatsoulas, C.; Anand, S. S. (2013). "Menopausal hormone therapy for the primary prevention of chronic conditions. U.S. Preventive Services Task Force Recommendation Statement" (pdf). Polskie Archiwum Medycyny Wewnetrznej. 123 (3): 112–117. PMID 23396275.
- 1 2 Nelson, H. D.; Walker, M.; Zakher, B.; Mitchell, J. (2012). "Menopausal hormone therapy for the primary prevention of chronic conditions: A systematic review to update the U.S. Preventive Services Task Force recommendations". Annals of Internal Medicine. 157 (2): 104–113. doi:10.7326/0003-4819-157-2-201207170-00466. PMID 22786830.
- 1 2 3 Santen, RJ; Utian, WH (2010). "Executive Summary: Postmenopausal Hormone Therapy: An Endocrine Society Scientific Statement". J Clin Endocrinol Metab. 95 S1–S66 (Supplement 1): s1–s66. doi:10.1210/jc.2009-2509. Retrieved Jan 16, 2015.
- ↑ Marjoribanks, J.; Farquhar, C.; Roberts, H.; Lethaby, A. (2012). Farquhar, Cindy, ed. "Long term hormone therapy for perimenopausal and postmenopausal women". The Cochrane Library. 7: CD004143. doi:10.1002/14651858.CD004143.pub4. PMID 22786488.
- ↑ "Medscape". www.medscape.com.
- ↑ "Hormone therapy for brain performance: No effect, whether started early or late". www.sciencedaily.com.
- ↑ Chlebowski, R. T.; Kuller, L. H.; Prentice, R. L.; Stefanick, M. L.; Manson, J. E.; Gass, M.; Aragaki, A. K.; Ockene, J. K.; Lane, D. S.; Sarto, G. E.; Rajkovic, A.; Schenken, R.; Hendrix, S. L.; Ravdin, P. M.; Rohan, T. E.; Yasmeen, S.; Anderson, G.; Whi, I. (2009). "Breast Cancer after Use of Estrogen plus Progestin in Postmenopausal Women". New England Journal of Medicine. 360 (6): 573–587. doi:10.1056/NEJMoa0807684. PMID 19196674.
- ↑ George, James L.; Colman, Robert W.; Goldhaber, Samuel Z.; Victor J. Marder (2006). Hemostasis and thrombosis: basic principles and clinical practice. Hagerstwon, MD: Lippincott Williams & Wilkins. pp. 1239. ISBN 0-7817-4996-4.
- ↑ Maryam Darabi, Mohsen Ani, Mojtaba Panjehpour, Mohammed Rabbani, Ahmad Movahedian & Elahe Zarean (January–February 2011). "Effect of estrogen receptor beta A1730G polymorphism on ABCA1 gene expression response to postmenopausal hormone replacement therapy". Genetic testing and molecular biomarkers. 15 (1-2): 11–15. doi:10.1089/gtmb.2010.0106. PMID 21117950.
- ↑ Chlebowski, R. T.; Anderson, G. L. (2015). "Menopausal hormone therapy and breast cancer mortality: clinical implications". Therapeutic Advances in Drug Safety. 6 (2): 45–56. doi:10.1177/2042098614568300. ISSN 2042-0986.
- ↑ Rossouw, J. E.; Prentice, R. L.; Manson, J. E.; Wu, L.; Barad, D.; Barnabei, V. M.; Ko, M.; Lacroix, A. Z.; Margolis, K. L.; Stefanick, M. L. (2007). "Postmenopausal Hormone Therapy and Risk of Cardiovascular Disease by Age and Years Since Menopause". JAMA: the Journal of the American Medical Association. 297 (13): 1465–1477. doi:10.1001/jama.297.13.1465. PMID 17405972.
- ↑ Bethea CL (Feb 2011). "MPA: Medroxy-Progesterone Acetate Contributes to Much Poor Advice for Women". Endocrinology. 152 (2): 343–345. doi:10.1210/en.2010-1376. PMC 3037166
 . PMID 21252179.
. PMID 21252179. - ↑ Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Miller VM, Naftolin F, Santoro N (March 2005). "KEEPS: The Kronos Early Estrogen Prevention Study". Climacteric. 8 (1): 3–12. doi:10.1080/13697130500042417. PMID 15804727.
- ↑ Langer RD (August 2010). "On the need to clarify and disseminate contemporary knowledge of hormone therapy initiated near menopause". Climacteric. 13 (4): 303–6. doi:10.3109/13697137.2010.496316. PMID 20540591.
- ↑ Studd J (March 2010). "Ten reasons to be happy about hormone replacement therapy: a guide for patients". Menopause Int. 16 (1): 44–6. doi:10.1258/mi.2010.010001. PMID 20424287.
- ↑ Salpeter, S. R.; Cheng, J.; Thabane, L.; Buckley, N. S.; Salpeter, E. E. (2009). "Bayesian Meta-analysis of Hormone Therapy and Mortality in Younger Postmenopausal Women". The American Journal of Medicine. 122 (11): 1016–1022.e1. doi:10.1016/j.amjmed.2009.05.021. PMID 19854329.
- ↑ Anderson, G. L.; Chlebowski, R. T.; Rossouw, J. E.; Rodabough, R. J.; McTiernan, A.; Margolis, K. L.; Aggerwal, A.; David Curb, J. D.; Hendrix, S. L.; Allan Hubbell, F. A.; Khandekar, J.; Lane, D. S.; Lasser, N.; Lopez, A. M.; Potter, J.; Ritenbaugh, C. (2006). "Prior hormone therapy and breast cancer risk in the Women's Health Initiative randomized trial of estrogen plus progestin". Maturitas. 55 (2): 103–115. doi:10.1016/j.maturitas.2006.05.004. PMID 16815651.
- ↑ Hulley, S.; Grady, D.; Bush, T.; Furberg, C.; Herrington, D.; Riggs, B.; Vittinghoff, E. (1998). "Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group". JAMA: the Journal of the American Medical Association. 280 (7): 605–613. doi:10.1001/jama.280.7.605. PMID 9718051.
- ↑ Salpeter, S. R.; Walsh, J. M. E.; Greyber, E.; Ormiston, T. M.; Salpeter, E. E. (2004). "Mortality associated with hormone replacement therapy in younger and older women". Journal of General Internal Medicine. 19 (7): 791–804. doi:10.1111/j.1525-1497.2004.30281.x. PMC 1492478
 . PMID 15209595.
. PMID 15209595. - ↑ Grodstein, F.; Stampfer, M. J.; Colditz, G. A.; Willett, W. C.; Manson, J. E.; Joffe, M.; Rosner, B.; Fuchs, C.; Hankinson, S. E.; Hunter, D. J.; Hennekens, C. H.; Speizer, F. E. (1997). "Postmenopausal Hormone Therapy and Mortality". New England Journal of Medicine. 336 (25): 1769–1775. doi:10.1056/NEJM199706193362501. PMID 9187066.
- ↑ Fraser IS, Mansour D (March 2006). "Delivery systems for hormone replacement therapy". Expert Opin Drug Deliv. 3 (2): 191–204. doi:10.1517/17425247.3.2.191. PMID 16506947.
- 1 2 3 Miller M.M.; Franklin K.B.J. (1999). "Theoretical basis for the benefit of postmenopausal estrogen substitution". Experimental Gerontology. 34: 587–604. doi:10.1016/S0531-5565(99)00032-7.
- ↑ Olié, V. R.; Canonico, M.; Scarabin, P. Y. (2010). "Risk of venous thrombosis with oral versus transdermal estrogen therapy among postmenopausal women". Current Opinion in Hematology. 17 (5): 457–463. doi:10.1097/MOH.0b013e32833c07bc. PMID 20601871.
- 1 2 Scarabin, P. Y.; Oger, E.; Plu-Bureau, G. V.; EStrogen THromboEmbolism Risk Study Group (2003). "Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk". The Lancet. 362 (9382): 428–432. doi:10.1016/S0140-6736(03)14066-4. PMID 12927428.
- ↑ Stramba-Badiale, M. (2009). "Postmenopausal hormone therapy and the risk of cardiovascular disease". Journal of Cardiovascular Medicine. 10 (4): 303–309. doi:10.2459/JCM.0b013e328324991c. PMID 19430340.
- 1 2 Anderson, G. L.; Limacher, M.; Assaf, A. R.; Bassford, T.; Beresford, S. A.; Black, H.; Bonds, D.; Brunner, R.; Brzyski, R.; Caan, B.; Chlebowski, R.; Curb, D.; Gass, M.; Hays, J.; Heiss, G.; Hendrix, S.; Howard, B. V.; Hsia, J.; Hubbell, A.; Jackson, R.; Johnson, K. C.; Judd, H.; Kotchen, J. M.; Kuller, L.; Lacroix, A. Z.; Lane, D.; Langer, R. D.; Lasser, N.; Lewis, C. E.; Manson, J. (2004). "Effects of Conjugated Equine Estrogen in Postmenopausal Women with Hysterectomy: The Women's Health Initiative Randomized Controlled Trial". JAMA: the Journal of the American Medical Association. 291 (14): 1701–1712. doi:10.1001/jama.291.14.1701. PMID 15082697.
- ↑ Manson, J. E.; Hsia, J.; Johnson, K. C.; Rossouw, J. E.; Assaf, A. R.; Lasser, N. L.; Trevisan, M.; Black, H. R.; Heckbert, S. R.; Detrano, R.; Strickland, O. L.; Wong, N. D.; Crouse, J. R.; Stein, E.; Cushman, M.; Women's Health Initiative Investigators (2003). "Estrogen plus Progestin and the Risk of Coronary Heart Disease". New England Journal of Medicine. 349 (6): 523–534. doi:10.1056/NEJMoa030808. PMID 12904517.
- ↑ Boardman, Henry MP; Hartley, Louise; Eisinga, Anne; Main, Caroline; Roqué i Figuls, Marta; Bonfill Cosp, Xavier; Gabriel Sanchez, Rafael; Knight, Beatrice; Boardman, Henry MP (2015). "Hormone therapy for preventing cardiovascular disease in post-menopausal women". Reviews. doi:10.1002/14651858.CD002229.pub4.
- ↑ Darabi, M.; Rabbani, M.; Ani, M.; Zarean, E.; Panjehpour, M.; Movahedian, A. (2011). "Increased leukocyte ABCA1 gene expression in post-menopausal women on hormone replacement therapy". Gynecological Endocrinology. 27 (9): 701–705. doi:10.3109/09513590.2010.507826. PMID 20807164.
- ↑ Young, Robert; Arlan F., Jr Fuller; Fuller, Arlan F.; Michael V. Seiden (2004). Uterine cancer. Hamilton, Ont: B.C. Decker. ISBN 1-55009-163-8.
- ↑ Copland, J. A.; Sheffield-Moore, M.; Koldzic-Zivanovic, N.; Gentry, S.; Lamprou, G.; Tzortzatou-Stathopoulou, F.; Zoumpourlis, V.; Urban, R. J.; Vlahopoulos, S. A. (2009). "Sex steroid receptors in skeletal differentiation and epithelial neoplasia: Is tissue-specific intervention possible?". BioEssays. 31 (6): 629–641. doi:10.1002/bies.200800138. PMID 19382224.
- ↑ Jeff Donn (2007-05-02). "Hormones may ward off dementia in women: Controversial treatment effective when taken soon after menopause". Associated Press.
- ↑ Schmidt, R; Fazekas, F; Reinhart, B; Kapeller, P; Fazekas, G; Offenbacher, H; Eber, B; Schumacher, M; Freidl, W.; et al. (1996). "Estrogen replacement therapy in older women: a neuropsychological and brain MRI study". J Am Geriatr Soc. 44 (11): 1307–13. PMID 8909345.
- ↑ Voytko, ML; Murray, R; Higgs, GJ. (2009). "Executive Function and Attention Are Preserved in Older Surgically Menopausal Monkeys Receiving Estrogen or Estrogen Plus Progesterone". Journal of Neuroscience. 29 (33): 10362–10370. doi:10.1523/JNEUROSCI.1591-09.2009. PMC 2744632
 . PMID 19692611.
. PMID 19692611. - ↑ Letendre, I.; Lopes, P. (2012). "Ménopause et risques carcinologiques". Journal de Gynécologie Obstétrique et Biologie de la Reproduction. 41 (7): F33–F37. doi:10.1016/j.jgyn.2012.09.006. PMID 23062839.
- ↑ Stefanick ML; Anderson GL; Margolis KL; et al. (2006). "Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy". JAMA. 295 (14): 1647–57. doi:10.1001/jama.295.14.1647. PMID 16609086.
- ↑ Hulley SB, Grady D (2004). "The WHI estrogen-alone trial--do things look any better?". JAMA. 291 (14): 1769–71. doi:10.1001/jama.291.14.1769. PMID 15082705.
- ↑ "Association between hormone replacement therapy use and breast cancer risk varies by race/ethnicity, body mass index, and breast density". JNCI Journal of the National Cancer Institute. 2013. doi:10.1093/jnci/djt264. ISSN 0027-8874.
- In turn citing: Hou, N.; Hong, S.; Wang, W.; Olopade, O. I.; Dignam, J. J.; Huo, D. (2013). "Hormone Replacement Therapy and Breast Cancer: Heterogeneous Risks by Race, Weight, and Breast Density". JNCI Journal of the National Cancer Institute. 105 (18): 1365–1372. doi:10.1093/jnci/djt207. ISSN 0027-8874.
- ↑ Fournier, A. S.; Berrino, F.; Clavel-Chapelon, F. O. (2007). "Unequal risks for breast cancer associated with different hormone replacement therapies: Results from the E3N cohort study". Breast Cancer Research and Treatment. 107 (1): 103–111. doi:10.1007/s10549-007-9523-x. PMC 2211383
 . PMID 17333341.
. PMID 17333341. - ↑ Kuhl, H.; Schneider, H. P. G. (2013). "Progesterone – promoter or inhibitor of breast cancer". Climacteric. 16 Suppl 1: 54–68. doi:10.3109/13697137.2013.768806. PMID 23336704.
- ↑ Management of the menopause after breast cancer, from The Royal Australian and New Zealand College of Obstetricians and Gynaecologists. College Statement C-Gyn 15. 1st Endorsed: February 2003. Current: November 2011. Review: November 2014
- ↑ Collaborative Group on Epidemiological Studies of Ovarian Cancer (12 February 2015). "Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies". The Lancet. doi:10.1016/S0140-6736(14)61687-1.
- 1 2 Levine, M (2016). "Menopause accelerates biological aging". Proc Natl Acad Sci USA: 201604558. PMID 27457926.
- ↑ Horvath S (2013). "DNA methylation age of human tissues and cell types". Genome Biology. 14: R115. doi:10.1186/gb-2013-14-10-r115. PMC 4015143
 . PMID 24138928.
. PMID 24138928. - ↑ Santoro, DeSoto, Jae Hong Lee (December 2010). "Hormone Therapy and Menopause". National Research Center for Women and Families.
- ↑ Gina Kolata (2007-04-19). "Sharp Drop in Rates of Breast Cancer Holds". New York Times.
- ↑ Roni Caryn Rabin (2007-08-28). "For a Low-Dose Hormone, Take Your Pick". New York Times. Many women seeking natural remedies have turned to compounding pharmacies, which use bioidentical hormones that are chemically synthesized but with the same molecular structure as hormones produced by a woman's body.
- ↑ John Gever (2011-04-05). "New WHI Estrogen Analysis Shows Lower Breast Ca Risk". MedPageToday.
- ↑ Boothby, L. A.; Doering, P. L.; Kipersztok, S. (2004). "Bioidentical hormone therapy: A review". Menopause. 11 (3): 356–367. doi:10.1097/01.GME.0000094356.92081.EF. PMID 15167316.
- ↑ "FDA Takes Action Against Compounded Menopause Hormone Therapy Drugs". FDA. 2008-01-09. Retrieved 2009-02-17.
- 1 2 3 4 5 6 7 Sarrel, P.M. (2000). Effects of hormone replacement therapy on sexual psychophysiology and behavior in postmenopause. Journal of Women’s Health and Gender-Based Medicine, 9, 25-32
- 1 2 3 4 5 6 Gonzalez, M., Viagara, G., Caba, F., & Molina, E. (2004). Sexual function, menopause and hormone replacement therapy (HRT). The European Menopause Journal, 48, 411-420. doi:10.1016/j.maturitas.2003.10.005
- 1 2 Nappi, R.E. et al., (2006). Clitoral stimulation in postmenopausal women with sexual dysfunction: A pilot randomized study with hormone therapy. European Menopause Journal, 55, 288-295
- 1 2 3 4 Moore, E., Wisniewski, & Dobs, A. (2003). Endocrine treatment of transsexual people: A review of treatment regimens, outcomes, and adverse effects. The Journal of Endocrinology & Metabolism, 88(9), 3467-3473. Doi: 10.1210/jc.2002-021967
- 1 2 3 4 Klein, C., & Gorzalka, B.B. (2009). Sexual functioning in transsexuals following hormone therapy and genital surgery: A review. Journal of Sexual Medicine, 6(11), 2922-2939. doi:10.1111/j.1743-6109.2009.01370.x
- ↑ Suchowersky O, Muthipeedika J (December 2005). "A case of late-onset chorea". Nat Clin Pract Neurol. 1 (2): 113–6. doi:10.1038/ncpneuro0052. PMID 16932507.
- ↑ "Combined Estrogen-Progestogen Menopausal Therapy". International Agency for Cancer Research. 91: 206.
- ↑ "HRT treatments". Health Express. Retrieved 2013-09-30.
- ↑ Estrogen (Vaginal Route) from Mayo Clinic / Thomson Healthcare Inc. Portions of this document last updated: Nov. 1, 2011