Heptacene
| | |
 | |
| Names | |
|---|---|
| IUPAC name
heptacene | |
| Identifiers | |
| 258-38-8 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 4574185 |
| PubChem | 5460712 |
| |
| |
| Properties | |
| C30H18 | |
| Molar mass | 378.47 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Heptacene is an organic compound and a polycyclic aromatic hydrocarbon and the seventh member of the acene or polyacene family of linear fused benzene rings.[1] This compound has long been pursued by chemists [2][3][4] because of its potential interest in electronic applications and was first synthesized but not isolated in 2006.[5][6]
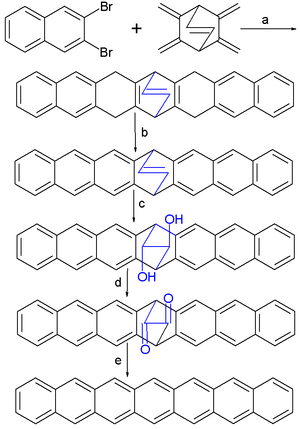
The final step is a photochemical decarbonylization with a 1,2-dione bridge extruded as carbon monoxide. In solution heptacene is not formed because it is very unstable being a reactive DA diene and quickly reacts with oxygen or forms dimers. When on the other hand the dione precursor is dissolved in a PMMA matrix first, heptacene can be studied by spectroscopy. Heptacene has been studied spectroscopically at cryogenic temperatures in a matrix.[7] When dissolved in sulfuric acid the heptacene dication is reported to be stable at room-temperature for more than a year in absence of oxygen.[8]
Derivatives
7,16-Bis(tris(trimethylsilyl)silylethynyl)heptacene was synthesised in 2005.[9] This compound is stable in the solid state for a week but decomposes in contact with air. Its synthesis started from anthraquinone and naphthalene-2,3-dicarboxaldehyde. More stable substituted heptacenes have been reported: with stabilizing p-(t-butyl)thiophenyl substituents[10] and with phenyl and triisopropylsilylethynyl groups.[11]
References
- ↑ Zade, Sanjio S.; Bendikov, Michael (2010). "Heptacene and Beyond: the Longest Characterized Acenes". Angewandte Chemie International Edition. 49 (24): n/a. doi:10.1002/anie.200906002. PMID 20468014.
- ↑ Clar, E. (1942), Heptacen ein einfacher, „ultragrüner” Kohlenwasserstoff (Aromatische Kohlenwasserstoffe, XXXV. Mitteil.). Ber. dtsch. Chem. Ges. A/B, 75: 1330–1338. doi:10.1002/cber.19420751114
- ↑ Cyclic Dienes. XI. New Syntheses of Hexacene and Heptacene William J. Bailey and Chien-Wei Liao Journal of the American Chemical Society 1955 77 (4), 992-993 doi:10.1021/ja01609a055
- ↑ 519. Four higher annellated pyrenes with acene character B. Boggiano and E. Clar J. Chem. Soc., 1957, 2681-2689 doi:10.1039/JR9570002681
- ↑ Photogeneration of Heptacene in a Polymer Matrix Rajib Mondal, Bipin K. Shah, and Douglas C. Neckers J. Am. Chem. Soc.; 2006; 128(30) pp 9612 - 9613; (Communication) doi:10.1021/ja063823i
- ↑ a| Diels-Alder reaction of dibromonaphthalene forming a naphthyne and bicyclo[2,2,2]oct-2,3,5,6,7-pentaene with n-butyllithium in toluene 3 hours at -50 to -60°C 53% chemical yield b| organic oxidation with P-chloranil in toluene 2 hours reflux and 81% yield c] Bishydroxylation with N-Methylmorpholine N-oxide and osmium tetroxide in acetone and t-butanol at room temperature for 48 hours, 83% yield d] Swern oxidation with trifluoroacetic acid in dimethyl sulfoxide and dichloromethane at -78°C, 51% yield e] photochemical decarbonylization in a PMMA matrix at 395 nm
- ↑ Synthesis, Stability, and Photochemistry of Pentacene, Hexacene, and Heptacene: A Matrix Isolation Study Rajib Mondal, Christina Tönshoff, Dmitriy Khon, Douglas C. Neckers, and Holger F. Bettinger Journal of the American Chemical Society 2009 131 (40), 14281-14289 doi:10.1021/ja901841c
- ↑ Einholz, R.; Bettinger, H. F. (2013). "Heptacene: Increased Persistence of a 4n+2 π-Electron Polycyclic Aromatic Hydrocarbon by Oxidation to the 4n π-Electron Dication". Angew. Chem. Int. Ed. 52: 9818–9820. doi:10.1002/anie.201209722.
- ↑ Payne, Marcia M.; Parkin, Sean R.; Anthony, John E. (2005). "Functionalized Higher Acenes: Hexacene and Heptacene". Journal of the American Chemical Society. 127 (22): 8028–9. doi:10.1021/ja051798v. PMID 15926823.
- ↑ Kaur, Irvinder; Stein, Nathan N.; Kopreski, Ryan P.; Miller, Glen P. (2009). "Exploiting Substituent Effects for the Synthesis of a Photooxidatively Resistant Heptacene Derivative". Journal of the American Chemical Society. 131 (10): 3424–5. doi:10.1021/ja808881x. PMID 19243093.
- ↑ Chun, Doris; Cheng, Yang; Wudl, Fred (2008). "The Most Stable and Fully Characterized Functionalized Heptacene". Angewandte Chemie International Edition. 47 (44): 8380–5. doi:10.1002/anie.200803345. PMID 18825763.