Heat-stable enterotoxin
| Heat-stable enterotoxin B, secretory | |||||||||
|---|---|---|---|---|---|---|---|---|---|
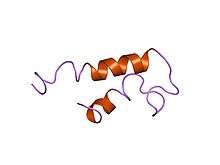 Structure of Escherichia coli heat-stable enterotoxin b.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | STb_secrete | ||||||||
| Pfam | PF09075 | ||||||||
| InterPro | IPR015160 | ||||||||
| PROSITE | PDOC00246 | ||||||||
| SCOP | 1ehs | ||||||||
| SUPERFAMILY | 1ehs | ||||||||
| OPM protein | 1ehs | ||||||||
| |||||||||
| Heat-stable enterotoxin ST | |||||||||
|---|---|---|---|---|---|---|---|---|---|
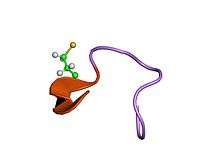 structural characteristics for biological activity of heat-stable enterotoxin produced by enterotoxigenic escherichia coli: x-ray crystallography of weakly toxic and nontoxic analogs | |||||||||
| Identifiers | |||||||||
| Symbol | Enterotoxin_ST | ||||||||
| Pfam | PF02048 | ||||||||
| InterPro | IPR001489 | ||||||||
| PROSITE | PDOC00246 | ||||||||
| SCOP | 1etn | ||||||||
| SUPERFAMILY | 1etn | ||||||||
| |||||||||
| Heat stable E.coli enterotoxin 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Enterotoxin_HS1 | ||||||||
| Pfam | PF08090 | ||||||||
| InterPro | IPR012557 | ||||||||
| |||||||||
Heat-stable enterotoxins (STs) are secretory peptides produced by some bacterial strains, such as enterotoxigenic Escherichia coli[2] which are in general toxic to animals.
These peptides keep their 3D structure and remain active at temperatures as high as 100 °C.
Function
Different STs recognize distinct receptors on the surface of animal cells and thereby affect different intracellular signaling pathways. For example, STa enterotoxins bind and activate membrane-bound guanylate cyclase, which leads to the intracellular accumulation of cyclic GMP and downstream effects on several signaling pathways.[3][4][5][6] These events lead to the loss of electrolytes and water from intestinal cells.
Heat-stable toxin 1 of entero-aggregative Escherichia coli (EAST1) is a small toxin. It is not, however, solely associated with entero-aggregative E. coli but also with many other diarrhoeic E. coli families. Some studies have established the role of EAST1 in some human outbreaks of diarrhoea. Isolates from farm animals have been shown to carry the astA gene coding for EAST1. However, the relation between the presence of EAST1 and disease is not conclusive.[7]
Structure
The mature STa protein from Escherichia coli, which is the cause of acute diarrhoea in infants and travellers in developing countries, is a 19-residue peptide containing three disulphide bridges that are functionally important. STa contains an N-terminal signal peptide composed of two domains, Pre and Pro, involved in extracellular toxin release, and a core enterotoxigenic domain.[8]
Members of heat-stable enterotoxin B family assume a helical secondary structure, with two alpha helices forming a disulfide cross-linked alpha-helical hairpin. The disulfide bonds are crucial for the toxic activity of the protein, and are required for maintenance of the tertiary structure, and subsequent interaction with the particulate form of guanylate cyclase, increasing cyclic GMP levels within the host intestinal epithelial cells.[9]
References
- ↑ Sukumar M, Rizo J, Wall M, Dreyfus LA, Kupersztoch YM, Gierasch LM (September 1995). "The structure of Escherichia coli heat-stable enterotoxin b by nuclear magnetic resonance and circular dichroism". Protein Sci. 4 (9): 1718–29. doi:10.1002/pro.5560040907. PMC 2143221
 . PMID 8528070.
. PMID 8528070. - ↑ Ghanekar Y, Chandrashaker A, Visweswariah SS (September 2003). "Cellular refractoriness to the heat-stable enterotoxin peptide is associated with alterations in levels of the differentially glycosylated forms of guanylyl cyclase C". Eur. J. Biochem. 270 (18): 3848–57. doi:10.1046/j.1432-1033.2003.03779.x. PMID 12950269.
- ↑ Hasegawa M, Shimonishi Y (February 2005). "Recognition and signal transduction mechanism of Escherichia coli heat-stable enterotoxin and its receptor, guanylate cyclase C". J. Pept. Res. 65 (2): 261–71. doi:10.1111/j.1399-3011.2005.00218.x. PMID 15705168.
- ↑ Al-Majali AM, Asem EK, Lamar CH, Robinson JP, Freeman MJ, Saeed AM (June 2000). "Characterization of the interaction of Escherichia coli heat-stable enterotoxin (STa) with its putative receptor on the intestinal tract of newborn calves". FEMS Immunol. Med. Microbiol. 28 (2): 97–104. doi:10.1111/j.1574-695x.2000.tb01462.x. PMID 10799798.
- ↑ Al-Majali AM, Ababneh MM, Shorman M, Saeed AM (February 2007). "Interaction of Escherichia coli heat-stable enterotoxin (STa) with its putative receptor on the intestinal tract of newborn kids". FEMS Immunol. Med. Microbiol. 49 (1): 35–40. doi:10.1111/j.1574-695X.2006.00167.x. PMID 17094787.
- ↑ Giannella RA, Mann EA (2003). "E. coli heat-stable enterotoxin and guanylyl cyclase C: new functions and unsuspected actions". Trans. Am. Clin. Climatol. Assoc. 114: 67–85; discussion 85–6. PMC 2194511
 . PMID 12813912.
. PMID 12813912. - ↑ Veilleux S, Dubreuil JD (2006). "Presence of Escherichia coli carrying the EAST1 toxin gene in farm animals". Vet. Res. 37 (1): 3–13. doi:10.1051/vetres:2005045. PMID 16336921.
- ↑ Sato T, Shimonishi Y (March 2004). "Structural features of Escherichia coli heat-stable enterotoxin that activates membrane-associated guanylyl cyclase". J. Pept. Res. 63 (3): 200–6. doi:10.1111/j.1399-3011.2004.00125.x. PMID 15049831.
- ↑ Rizo J, Gierasch LM, Sukumar M, Wall M, Dreyfus LA, Kupersztoch YM (1995). "The structure of Escherichia coli heat-stable enterotoxin b by nuclear magnetic resonance and circular dichroism". Protein Sci. 4 (9): –. doi:10.1002/pro.5560040907. PMC 2143221
 . PMID 8528070.
. PMID 8528070.
This article incorporates text from the public domain Pfam and InterPro IPR001489
This article incorporates text from the public domain Pfam and InterPro IPR015160
This article incorporates text from the public domain Pfam and InterPro IPR012557