Harry Anderson (chemist)
| Harry Anderson | |
|---|---|
| Born |
Harry Laurence Anderson January 12, 1964 |
| Nationality | United Kingdom |
| Fields | Organic chemistry |
| Institutions | |
| Alma mater | |
| Thesis | Model enzymes based on porphyrins (1991) |
| Doctoral advisor | Jeremy Sanders |
| Known for | Porphyrin Nanorings |
| Notable awards | FRS (2013)[1] |
|
Website hla | |
Harry Laurence Anderson, FRS is a British chemist in Department of Chemistry, University of Oxford. He is well known for his contributions in the syntheses of supramolecular systems (porphyrin nanorings and nanowires) and exploration of the extraordinary physical properties of the large pi-conjugate systems. He is currently professor of chemistry at Keble College, Oxford.
Biography
Harry Anderson studied chemistry at University of Oxford, where he received Bachelor of Arts degree in 1987. He continued his study at University of Cambridge, supervised by Prof. Jeremy Sanders FRS, and received his doctoral degree in 1990. He started his independent research as a research fellow at Magdalene College, Cambridge in 1990-1993, and conducted his research in 1993-1994 as SERC postdoctoral research fellow at ETH-Zürich, Switzerland. He returned to University of Oxford in 1994 as university lecturer in organic chemistry and tutor in Keble College. In 2004, he became professor of chemistry at the University of Oxford. In May 2013, he was elected as Fellow of the Royal Society.[2]
Research Interests
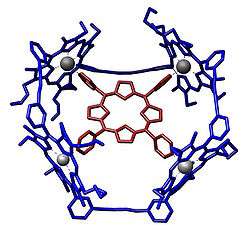
Template directed syntheses ubiquitously exist in nature (protein biosynthesis, etc.), which provides inspiration for synthesizing artificial supramolecular systems. Using porphyrin monomers/oligomers and molecular templates of various sizes, porphyrin nanoring systems can be constructed with high versatility.[5][6] These supramolecular systems also bear appealing coordination properties, providing inspirations for the coordination phenomena existing in nature.[7][8]
Vernier templating refers to the syntheses of complexes using templates and molecular building blocks with mismatching coordination numbers in order to construct larger molecular systems by incorporating more than one template molecule and more molecular building blocks than usual. Porphyrin nanoring systems are excellent examples in realizing this methodology and giant artificial molecular systems with their molecular weights of small proteins can be constructed.[9][10]
Based on the work of organic synthesis, his research interests have found wide range of collaborators from versatile academic backgrounds all over the world. It was found that elongated/encapsulated pi-conjugate systems constructed by porphyrins showed unprecedented physical properties in charge transfer,[11][12] two-photon absorption,[13] etc., thereby providing physicists and photobiologists new candidates and inspirations in their research.
Honours and Awards
- 2013 Fellow of The Royal Society[1]
- 2008 Merck-Karl Pfister Visiting Professor in Organic Chemistry, MIT, USA
- 2006 RSC Industrially-Sponsored Award for Supramolecular Chemistry[14]
- 2003 Royal Society of Chemistry Award for Materials Chemistry[14]
- 2003 Bob Hay Lecturer (Royal Society of Chemistry, Macrocyclic Chemistry)[15]
- 2001 Corday-Morgan Award (Royal Society of Chemistry)[16]
- 1995 Nuffield Foundation Award to Newly Appointed Science Lecturers[17]
- 1993 NATO/SERC Research Fellowship
Anderson's nomination for the Royal Society in 2013 reads:
| “ | Harry Anderson is known internationally for his insightful contributions to the design and synthesis of supramolecular materials and molecular wires. He has introduced new concepts for molecular design, and ground-breaking approaches to template-directed synthesis, leading to materials with unprecedented electronic and nonlinear optical characteristics. He has pioneered the investigation of conjugated porphyrin oligomers, encapsulated pi-systems, nanorings and two-photon absorbing dyes, and he has worked closely with physicists and photobiologists to understand the relationship between molecular structure and function. His work has resulted in profound insights into the factors controlling long-range electronic coupling and charge-transport in supramolecular systems.[1] | ” |
References
- 1 2 3 "Library and Archive Catalogue". London: The Royal Society. Archived from the original on 2014-04-25.
- ↑ Professor Harry Anderson FRS
- ↑ Anderson, S.; Anderson, H. L.; Bashall, A.; McPartlin, M.; Sanders, J. K. M. (1995). "Assembly and Crystal Structure of a Photoactive Array of Five Porphyrins". Angewandte Chemie International Edition in English. 34 (10): 1096–1099. doi:10.1002/anie.199510961.
- ↑ Stanier, C. A.; o’Connell, M. J.; Anderson, H. L.; Clegg, W. (2001). "Synthesis of fluorescent stilbene and tolan rotaxanes by Suzuki coupling". Chemical Communications (5): 493–494. doi:10.1039/b010015n.
- ↑ Hoffmann, M.; Kärnbratt, J.; Chang, M. H.; Herz, L. M.; Albinsson, B.; Anderson, H. L. (2008). "Enhanced π Conjugation around a Porphyrin[6] Nanoring". Angewandte Chemie International Edition. 47 (27): 4993–4996. doi:10.1002/anie.200801188.
- ↑ Hoffmann, M.; Wilson, C. J.; Odell, B.; Anderson, H. L. (2007). "Template-Directed Synthesis of a π-Conjugated Porphyrin Nanoring". Angewandte Chemie International Edition. 46 (17): 3122–3125. doi:10.1002/anie.200604601.
- ↑ Hogben, H. J.; Sprafke, J. K.; Hoffmann, M.; Pawlicki, M. O.; Anderson, H. L. (2011). "Stepwise Effective Molarities in Porphyrin Oligomer Complexes: Preorganization Results in Exceptionally Strong Chelate Cooperativity". Journal of the American Chemical Society. 133 (51): 20962–20969. doi:10.1021/ja209254r.
- ↑ Sprafke, J. K.; Odell, B.; Claridge, T. D. W.; Anderson, H. L. (2011). "All-or-Nothing Cooperative Self-Assembly of an Annulene Sandwich". Angewandte Chemie International Edition. 50 (24): 5572–5575. doi:10.1002/anie.201008087.
- ↑ o’Sullivan, M. C.; Sprafke, J. K.; Kondratuk, D. V.; Rinfray, C.; Claridge, T. D. W.; Saywell, A.; Blunt, M. O.; o’Shea, J. N.; Beton, P. H.; Malfois, M.; Anderson, H. L. (2011). "Vernier templating and synthesis of a 12-porphyrin nano-ring". Nature. 469 (7328): 72–75. doi:10.1038/nature09683.
- ↑ Kondratuk, D. V.; Perdigao, L. M. A.; O'Sullivan, M. C.; Svatek, S.; Smith, G.; O'Shea, J. N.; Beton, P. H.; Anderson, H. L. (2012). "Two Vernier-Templated Routes to a 24-Porphyrin Nanoring". Angewandte Chemie International Edition. 51 (27): 6696–6699. doi:10.1002/anie.201202870.
- ↑ Sedghi, G.; García-Suárez, V. C. M.; Esdaile, L. J.; Anderson, H. L.; Lambert, C. J.; Martín, S.; Bethell, D.; Higgins, S. J.; Elliott, M.; Bennett, N.; MacDonald, J. E.; Nichols, R. J. (2011). "Long-range electron tunnelling in oligo-porphyrin molecular wires". Nature Nanotechnology. 6 (8): 517–23. doi:10.1038/nnano.2011.111. PMID 21804555.
- ↑ López-Duarte, I.; Reeve, J. E.; Pérez-Moreno, J.; Boczarow, I.; Depotter, G.; Fleischhauer, J.; Clays, K.; Anderson, H. L. (2013). ""Push-no-pull" porphyrins for second harmonic generation imaging". Chemical Science. 4 (5): 2024. doi:10.1039/C3SC22306J.
- ↑ Odom, S. A.; Webster, S.; Padilha, L. A.; Peceli, D.; Hu, H.; Nootz, G.; Chung, S. J.; Ohira, S.; Matichak, J. D.; Przhonska, O. V.; Kachkovski, A. D.; Barlow, S.; BréDas, J. L.; Anderson, H. L.; Hagan, D. J.; Van Stryland, E. W.; Marder, S. R. (2009). "Synthesis and Two-Photon Spectrum of a Bis(Porphyrin)-Substituted Squaraine". Journal of the American Chemical Society. 131 (22): 7510–7511. doi:10.1021/ja901244e.
- 1 2
- ↑ Bob Hay Lectureship, RSC
- ↑ RSC award archive
- ↑ Nuffield Foundation