Haber process

The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today.[1] It is named after its inventors, the German chemists Fritz Haber and Carl Bosch, who developed it in the first half of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using a metal catalyst under high temperatures and pressures:
- N2 + 3 H2 → 2 NH3 (ΔH° = −91.8 kJ) => (ΔH° = −45.8 kJ·mol−1)
Before the development of the Haber process, ammonia had been difficult to produce on an industrial scale[2][3][4] with early methods such as the Birkeland–Eyde process and Frank–Caro process all being highly inefficient.
Although the Haber process is mainly used to produce fertilizer today, during World War I it provided Germany with a source of ammonia for the production of explosives, compensating for the Allied trade blockade on Chilean saltpeter.
History
Throughout the 19th century the demand for nitrates and ammonia for use as fertilizers and industrial feedstocks had been steadily increasing. The main source was mining niter deposits. At the beginning of the 20th century it was being predicted that these reserves could not satisfy future demand[5] and research into new potential sources of ammonia became more important. The obvious source was atmospheric nitrogen (N2), comprising nearly 80% of the air, however N2 is exceptionally stable and will not readily react with other chemicals. Converting N2 into ammonia posed a chemical challenge for chemists globally.
Haber, with his assistant Robert Le Rossignol, developed the high-pressure devices and catalysts needed to demonstrate the Haber process at laboratory scale.[6][7] They demonstrated their process in the summer of 1909 by producing ammonia from air, drop by drop, at the rate of about 125 ml (4 US fl oz) per hour. The process was purchased by the German chemical company BASF, which assigned Carl Bosch the task of scaling up Haber's tabletop machine to industrial-level production.[3][8] He succeeded in 1910. Haber and Bosch were later awarded Nobel prizes, in 1918 and 1931 respectively, for their work in overcoming the chemical and engineering problems of large-scale, continuous-flow, high-pressure technology.[9]
Ammonia was first manufactured using the Haber process on an industrial scale in 1913 in BASF's Oppau plant in Germany, reaching 20 tonnes per day the following year.[10]
During World War I, the production of munitions required large amounts of nitrate. The Allies had access to large sodium nitrate deposits in Chile (so called "Chile saltpetre") controlled by British companies. Germany had no such resources, so the Haber process proved essential to the German war effort.[9][11] Synthetic ammonia from the Haber process was used for the production of nitric acid, a precursor to the nitrates used in explosives.
Process

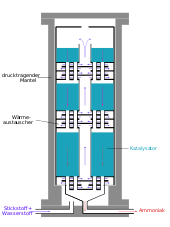
This conversion is typically conducted at 15–25 MPa (150–250 bar; 2,200–3,600 psi) and between 400–500 °C (752–932 °F), as the gases (nitrogen and hydrogen) are passed over four beds of catalyst, with cooling between each pass so as to maintain a reasonable equilibrium constant. On each pass only about 15% conversion occurs, but any unreacted gases are recycled, and eventually an overall conversion of 97% is achieved.[1]
The steam reforming, shift conversion, carbon dioxide removal, and methanation steps each operate at pressures of about 2.5–3.5 MPa (25–35 bar; 360–510 psi), and the ammonia synthesis loop operates at pressures ranging from 6–18 MPa (60–180 bar; 870–2,610 psi), depending upon which proprietary process is used.[1]
Sources of hydrogen
The major source of hydrogen is methane from natural gas. The conversion, steam reforming, is conducted with steam in a high temperature and pressure tube inside a reformer with a nickel catalyst, separating the carbon and hydrogen molecules in the natural gas.
Reaction rate and equilibrium
Nitrogen (N2) is very unreactive because the molecules are held together by strong triple bonds. The Haber process relies on catalysts that accelerate the scission of this triple bond.
Two opposing considerations are relevant to this synthesis: the position of the equilibrium and the rate of reaction. At room temperature, the equilibrium is strongly in favor of ammonia, but the reaction doesn't proceed at a detectable rate. The obvious solution is to raise the temperature, but because the reaction is exothermic, the equilibrium constant (using bar or atm units) becomes 1 around 150–200 °C (302–392 °F). (See Le Châtelier's principle.)
| Temperature (°C) | Kp |
|---|---|
| 300 | 4.34 × 10−3 |
| 400 | 1.64 × 10−4 |
| 450 | 4.51 × 10−5 |
| 500 | 1.45 × 10−5 |
| 550 | 5.38 × 10−6 |
| 600 | 2.25 × 10−6 |
Above this temperature, the equilibrium quickly becomes quite unfavorable at atmospheric pressure, according to the Van 't Hoff equation. Thus one might suppose that a low temperature is to be used and some other means to increase rate. However, the catalyst itself requires a temperature of at least 400 °C to be efficient.
Pressure is the obvious choice to favor the forward reaction because there are 4 moles of reactant for every 2 moles of product (see entropy), and the pressure used (15–25 MPa (150–250 bar; 2,200–3,600 psi)) alters the equilibrium concentrations to give a profitable yield.
Economically, pressure is an expensive commodity. Pipes and reaction vessels need to be strengthened, valves more rigorous, and there are safety considerations of working at 20 MPa. In addition, running pumps and compressors takes considerable energy. Thus the compromise used gives a single pass yield of around 15%.
Another way to increase the yield of the reaction would be to remove the product (i.e. ammonia gas) from the system. In practice, gaseous ammonia is not removed from the reactor itself, since the temperature is too high; it is removed from the equilibrium mixture of gases leaving the reaction vessel. The hot gases are cooled enough, whilst maintaining a high pressure, for the ammonia to condense and be removed as liquid. Unreacted hydrogen and nitrogen gases are then returned to the reaction vessel to undergo further reaction.
Catalysts
The most popular catalysts are based on iron promoted with K2O, CaO, SiO2, and Al2O3. The original Haber–Bosch reaction chambers used osmium as the catalyst, but it was available in extremely small quantities. Haber noted uranium was almost as effective and easier to obtain than osmium. Under Bosch's direction in 1909, the BASF researcher Alwin Mittasch discovered a much less expensive iron-based catalyst, which is still used today. Some ammonia production utilizes ruthenium-based catalysts (the KAAP process). Ruthenium forms more active catalysts that allows milder operating pressures. Such catalysts are prepared by decomposition of triruthenium dodecacarbonyl on graphite.[1]
In industrial practice, the iron catalyst is obtained from finely ground iron powder, which is usually obtained by reduction of high purity magnetite (Fe3O4). The pulverized iron metal is burnt (oxidized) to give magnetite of a defined particle size. The magnetite particles are then partially reduced, removing some of the oxygen in the process. The resulting catalyst particles consist of a core of magnetite, encased in a shell of wüstite (FeO), which in turn is surrounded by an outer shell of iron metal. The catalyst maintains most of its bulk volume during the reduction, resulting in a highly porous high surface area material, which enhances its effectiveness as a catalyst. Other minor components of the catalyst include calcium and aluminium oxides, which support the iron catalyst and help it maintain its surface area. These oxides of Ca, Al, K, and Si are unreactive to reduction by the hydrogen.[1]
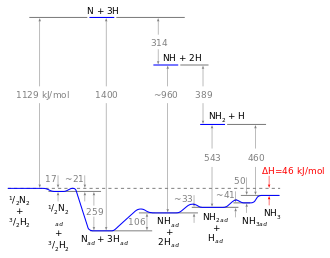
The reaction mechanism, involving the heterogeneous catalyst, is believed to involve the following steps:[13]
- N2 (g) → N2 (adsorbed)
- N2 (adsorbed) → 2 N (adsorbed)
- H2 (g) → H2 (adsorbed)
- H2 (adsorbed) → 2 H (adsorbed)
- N (adsorbed) + 3 H(adsorbed)→ NH3 (adsorbed)
- NH3 (adsorbed) → NH3 (g)
Reaction 5 occurs in three steps, forming NH, NH2, and then NH3. Experimental evidence points to reaction 2 as being the slow, rate-determining step. This is not unexpected since the bond broken, the nitrogen triple bond, is the strongest of the bonds that must be broken.
A major contributor to the elucidation of this mechanism is Gerhard Ertl.[14]
Economic and environmental aspects

When it was first invented, the Haber process needed to compete against another industrial process, the Cyanamide process. However, the Cyanamide process consumed large amounts of electrical power and was more labor-intensive than the Haber process.[9]:137–143
The Haber process now produces 450 million tonnes of nitrogen fertilizer per year, mostly in the form of anhydrous ammonia, ammonium nitrate, and urea. Three to five percent of the world's natural gas production is consumed in the Haber process (around 1–2% of the world's annual energy supply).[15][16][17][18] In combination with pesticides, these fertilizers have quadrupled the productivity of agricultural land:
- With average crop yields remaining at the 1900 level the crop harvest in the year 2000 would have required nearly four times more land and the cultivated area would have claimed nearly half of all ice-free continents, rather than under 15% of the total land area that is required today.[19]
Due to its dramatic impact on the human ability to grow food, the Haber process served as the "detonator of the population explosion", enabling the global population to increase from 1.6 billion in 1900 to today's 7 billion.[20] Nearly 80% of the nitrogen found in human tissues originated from the Haber-Bosch process.[21] Since nitrogen use efficiency is typically less than 50%,[22] our heavy use of industrial nitrogen fixation is disruptive to our biological habitat.[21][2]
See also
References
- 1 2 3 4 5 Appl, Max (2005), "Ammonia", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH
- 1 2 Smil, Vaclav (2004). Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production (1st ed.). Cambridge, MA: MIT. ISBN 9780262693134.
- 1 2 Hager, Thomas (2008). The Alchemy of Air: A Jewish genius, a doomed tycoon, and the scientific discovery that fed the world but fueled the rise of Hitler (1st ed.). New York, NY: Harmony Books. ISBN 978-0-307-35178-4.
- ↑ Sittig, Marshall (1979). Fertilizer Industry: Processes, Pollution Control, and Energy Conservation. Park Ridge, NJ: Noyes Data Corp. ISBN 0-8155-0734-8.
- ↑ James, Laylin K. (1993). Nobel Laureates in Chemistry 1901–1992 (3rd ed.). Washington,DC: American Chemical Society. p. 118. ISBN 0-8412-2690-3.
- ↑ Haber, Fritz (2012). Thermodynamik technischer Gasreaktionen (in German) (1st ed.). Paderborn: Salzwasser Verlag. ISBN 9783864448423.
- ↑ "Robert Le Rossignol, 1884–1976: Professional Chemist" (PDF), ChemUCL Newsletter, UCL Department of Chemistry: 8, 2009
- ↑ Patent US 990191
- 1 2 3 Hager, T. (2008). The Alchemy of Air. New York, NY: Harmony Books.
- ↑ Philip & Phyllis Morris, "From Fertile Minds" (review) American Scientist, 2001
- ↑ "Nobel Award to Haber". New York Times. 3 February 1920. Retrieved 11 October 2010.
- ↑ Brown, Theodore L.; LeMay, H. Eugene, Jr; Bursten, Bruce E (2006). "Table 15.2". Chemistry: The Central Science (10th ed.). Upper Saddle River, NJ: Pearson. ISBN 0-13-109686-9.
- ↑ Wennerström, Håkan; Lidin, Sven. "Scientific Background on the Nobel Prize in Chemistry 2007 Chemical Processes on Solid Surfaces" (PDF). NobelPrize.org. Swedish Academy of Sciences. Retrieved 2015-09-17.
- ↑ Bozso, F.; Ertl, G.; Grunze, M.; Weiss, M. (1977). "Interaction of nitrogen with iron surfaces: I. Fe(100) and Fe(111)". J. Catal. 49 (1): 18–41. doi:10.1016/0021-9517(77)90237-8.. Imbihl, R.; Behm, R. J.; Ertl, G.; Moritz, W. (1982). "The structure of atomic nitrogen adsorbed on Fe(100)". Surf. Sci. 123 (1): 129–140. Bibcode:1982SurSc.123..129I. doi:10.1016/0039-6028(82)90135-2.. Ertl, G.; Lee, S. B.; Weiss, M. (1982). "Kinetics of nitrogen adsorption on Fe(111)". Surf. Sci. 114 (2–3): 515–526. Bibcode:1982SurSc.114..515E. doi:10.1016/0039-6028(82)90702-6.. Ertl, G. (1983). "Primary steps in catalytic synthesis of ammonia". J. Vac. Sci. Tech. A. 1 (2): 1247–1253. doi:10.1116/1.572299.
- ↑ Smil, Vaclav (2004). Enriching the Earth: Fritz Haber, Carl Bosch, and the Transformation of World Food Production. Cambridge, MA: MIT Press. ISBN 9780262693134.
- ↑ "International Energy Outlook 2007".
- ↑ Fertilizer statistics. "?".
- ↑ Smith, Barry E. (September 2002). "Structure. Nitrogenase reveals its inner secrets". Science. 297 (5587): 1654–5. doi:10.1126/science.1076659. PMID 12215632.
- ↑ Smil, Vaclav (2011). "Nitrogen cycle and world food production" (PDF). World Agriculture. 2: 9–1.
- ↑ Smil, Vaclav (1999). "Detonator of the population explosion" (PDF). Nature. 400: 415. doi:10.1038/22672.
- 1 2 Howarth, R. W. (2008). "Coastal nitrogen pollution: a review of sources and trends globally and regionally". Harmful Algae. 8: 14–20. doi:10.1016/j.hal.2008.08.015.
- ↑ Oenema, O.; Witzke, H.P.; Klimont, Z.; Lesschen, J.P.; Velthof, G.L. (2009). "Integrated assessment of promising measures to decrease nitrogen losses in agriculture in EU-27". Agriculture, Ecosystems and Environment. 133: 280–288. doi:10.1016/j.agee.2009.04.025.
- "The Haber Process". Chemguide.co.uk.
External links
- Haber-Bosch process, most important invention of the 20th century, according to V. Smil, Nature, July 29, 1999, p 415 (by Jürgen Schmidhuber)
- Britannica guide to Nobel Prizes: Fritz Haber
- Nobel e-Museum - Biography of Fritz Haber
- BASF - Fertilizer out of thin air
- Uses and Production of Ammonia
- Haber Process for Ammonia Synthesis
- Review of "Between Genius and Genocide: The Tragedy of Fritz Haber, Father of Chemical Warfare" by Daniel Charles