HBx
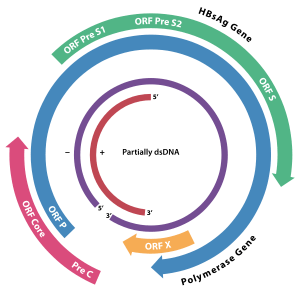
HBx is a hepatitis B viral protein.[1][2] It is 154 amino acids long and interferes with transcription, signal transduction, cell cycle progress, protein degradation, apoptosis and chromosomal stability in the host. It forms a heterodimeric complex with its cellular target protein (HBX interacting protein: HBXIP), and this interaction dysregulates centrosome dynamics and mitotic spindle formation.[3] It interacts with DDB1 (Damaged DNA Binding Protein 1) redirecting the ubiquitin ligase activity of the CUL4-DDB1 E3 complexes, which are intimately involved in the intracellular regulation of DNA replication and repair, transcription and signal transduction.[4]
Although Protein X is normally absent in the Avihepadnavirus, a vestigial version has been identified in the duck hepatitis virus genome.[5]
Although it lacks significant sequence identity with any known vertebrate proteins, it seems likely that it evolved from a DNA glycosylase.[6]
Transgenic mice expressing the X protein in liver are more likely than the wild type to develop hepatocellular carcinoma. This is because the X protein promotes cell cycle progression while binding to and inhibiting tumor suppressor protein p53 from performing their role. Experimental observations also suggest that HBx protein increases TERT and telomerase activity, prolonging the lifespan of hepatocytes and contributing to malignant transformation.[7]
Relation to PRMT1
In a study purifying cancerous liver cells infected with HBV, the level of expression of protein arginine methyltransferase 1 (PRMT1) was found to be associated with changes in transcription due to the methyltransferase function of PRMT1. Overexpression causes a reduction in the number of HBV genes transcribed, while conversely, underexpression causes an increase. PRMT1 was also found to be recruited by HBV DNA during the replication process to regulate the transcription process. Increased HBx expression in turn leads to an inhibition of PRMT1-mediated protein methylation, benefiting viral replication.[8]
References
- ↑ McClain SL, Clippinger AJ, Lizzano R, Bouchard MJ (November 2007). "Hepatitis B virus replication is associated with an HBx-dependent mitochondrion-regulated increase in cytosolic calcium levels". J. Virol. 81 (21): 12061–5. doi:10.1128/JVI.00740-07. PMC 2168786
 . PMID 17699583.
. PMID 17699583. - ↑ Bouchard MJ, Puro RJ, Wang L, Schneider RJ (July 2003). "Activation and inhibition of cellular calcium and tyrosine kinase signaling pathways identify targets of the HBx protein involved in hepatitis B virus replication". J. Virol. 77 (14): 7713–9. doi:10.1128/JVI.77.14.7713-7719.2003. PMC 161925
 . PMID 12829810.
. PMID 12829810. - ↑ Wen, Y.; Golubkov, VS.; Strongin, AY.; Jiang, W; Reed, J.C. (2008). "Interaction of hepatitis B viral oncoprotein with cellular target HBXIP dysregulates centrosome dynamics and mitotic spindle formation". Journal of Biological Chemistry. 283 (5): 2793–2803. doi:10.1074/jbc.M708419200. PMID 18032378.
- ↑ Li, T; Robert, E.I.; van Breugel, P.C.; Strubin, M.; Zheng, N. (2010). "A promiscuous alpha-helical motif anchors viral hijackers and substrate receptors to the CUL4-DDB1 ubiquitin ligase machinery". Nature Structural & Molecular Biology. Nature. 17 (1): 105–111. doi:10.1038/nsmb.1719. PMC 2823288
 . PMID 19966799.
. PMID 19966799. - ↑ Lin, B.; Anderson, D.A. (2000). "A vestigial X open reading frame in duck hepatitis B virus". Intervirology. Karger. 43 (3): 185–190. doi:10.1159/000025037. PMID 11044813.
- ↑ van Hemert, F.J.; van de Klundert, M.A.; Lukashov, V.V.; Kootstra, N.A.; Berkhout, B.; Zaaijer, H.L. (2011). "Protein x of hepatitis B virus: origin and structure similarity with the central domain of DNA glycosylase". PLoS ONE. 6 (8): e23392. doi:10.1371/journal.pone.0023392. PMC 3153941
 . PMID 21850270.
. PMID 21850270. - ↑ Kew, M.C. (2011). "Hepatitis B virus x protein in the pathogenesis of hepatitis B virus-induced hepatocellular carcinoma". Journal of Gastroenterolology and Hepatology. 26 (Suppl 1): 144–152. doi:10.1111/j.1440-1746.2010.06546.x. PMID 21199526.
- ↑ Benhenda S; Ducroux A; Rivière L; Sobhian B; Ward MD; Dion S; Hantz O; Protzer U; Michel ML; Benkirane M; Semmes OJ; Buendia MA; Neuveut C (2013). "Methyltransferase PRMT1 Is a Binding Partner of HBx and a Negative Regulator of Hepatitis B Virus Transcription". Journal of Virology. American Society for Microbiology. 87 (8): 4360–4371. doi:10.1128/JVI.02574-12. PMC 3624337
 . PMID 23388725.
. PMID 23388725.