HBTU
 | |
| Names | |
|---|---|
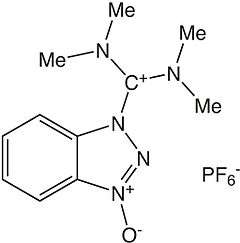
| IUPAC name
3-[Bis(dimethylamino)methyliumyl]-3H-benzotriazol-1-oxide hexafluorophosphate | |
| Other names
HBTU | |
| Identifiers | |
| 94790-37-1 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 2014894 |
| ECHA InfoCard | 100.133.815 |
| PubChem | 2733084 |
| |
| |
| Properties | |
| C11H16F6N5OP | |
| Molar mass | 379.25 g·mol−1 |
| Appearance | White crystals |
| Melting point | 200 °C (392 °F; 473 K) |
| Hazards | |
| Main hazards | Irritant |
| R-phrases | R36/37/38-R42/43 |
| S-phrases | S22-S26-S36/37/39 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
HBTU (2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate) is a coupling reagent used in solid phase peptide synthesis. It was introduced in 1978 and shows resistance against racemization.[1] It is used because of its mild activating properties.[2]
The product obtained by reaction of HOBt with tetramethyl chloro uronium salt (TMUCl) was assigned to a uronium type structure, presumably by analogy with the corresponding phosphonium salts, which bear a positive carbon atom instead of the phosphonium residue. Later, it was shown by X-ray analysis that salts crystallize as aminium rather than the corresponding uronium salts [3] .[4]
See also
References
- ↑ Knorr, R.; Trzeciak, A.; Bannwarth, W.; Gillessen, D. (1989). "New coupling reagents in peptide chemistry". Tetrahedron Letters. 30 (15): 1927–1930. doi:10.1016/S0040-4039(00)99616-3.
- ↑ Solange, A. (1992). "HBTU: a mild activating agent of muramic acid". Bioorganic & Medicinal Chemistry Letters. 2 (6): 571–574. doi:10.1016/S0960-894X(01)81199-9.
- ↑ Carpino, L.; Imazumi, H.; El-Faham, A.; Ferrer, F.; Zhang, C.; Lee, Y.; Foxman, B.; Henklein, P.; Hanay, C.; Mügge, C.; Wenschuh, H.; Klose, J.; Beyermann, M.; Bienert, M. (2002). "The uronium/guanidinium peptide coupling reagents: Finally the true uronium salts". Angewandte Chemie International Edition. 41 (3): 441–445. doi:10.1002/1521-3773(20020201)41:3<441::AID-ANIE441>3.0.CO;2-N.
- ↑ Abdelmoty, I.; Albericio, F.; Carpino, L.; Foxman, B.; Kates, S. (1994). "Structural studies of reagents for peptide bond formation: Crystal and molecular structures of HBTU and HATU". Letters in Peptide Science. 1 (2): 57–67. doi:10.1007/BF00126274.
This article is issued from Wikipedia - version of the 10/21/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.