Gyromitra esculenta
| Gyromitra esculenta | |
|---|---|
| | |
| Scientific classification | |
| Kingdom: | Fungi |
| Division: | Ascomycota |
| Class: | Pezizomycetes |
| Order: | Pezizales |
| Family: | Discinaceae |
| Genus: | Gyromitra |
| Species: | G. esculenta |
| Binomial name | |
| Gyromitra esculenta (Pers. ex Pers.) Fr. (1849) | |
| Synonyms[1] | |
| |
| Gyromitra esculenta | |
|---|---|
|
| |
| smooth hymenium | |
| cap is convex | |
| hymenium attachment is not applicable | |
| stipe is bare | |
|
spore print is yellow to buff | |
|
ecology is saprotrophic or mycorrhizal | |
|
edibility: choice or deadly | |
Gyromitra esculenta /ˌdʒaɪroʊˈmaɪtrə ˌɛskjəˈlɛntə, ˌdʒɪrə-/,[2] is an ascomycete fungus from the genus Gyromitra, widely distributed across Europe and North America. It normally fruits in sandy soils under coniferous trees in spring and early summer. The fruiting body, or mushroom, is an irregular brain-shaped cap dark brown in colour that can reach 10 cm (4 in) high and 15 cm (6 in) wide, perched on a stout white stipe up to 6 cm (2.4 in) high.
Although potentially fatal if eaten raw, Gyromitra esculenta is a popular delicacy in Scandinavia, Eastern Europe, and the upper Great Lakes region of North America. Although popular in some districts of the eastern Pyrenees, it is prohibited from sale to the public in Spain. It may be sold fresh in Finland, but it must be accompanied by warnings and instructions on correct preparation.
Although it is still commonly parboiled before preparation, evidence suggests that even this procedure may not make Gyromitra esculenta entirely safe for consumption.[3] When consumed, the principal active agent, gyromitrin, is hydrolyzed into the toxic compound monomethylhydrazine (MMH). The toxin affects the liver, central nervous system, and sometimes the kidneys. Symptoms of poisoning involve vomiting and diarrhea several hours after consumption, followed by dizziness, lethargy and headache. Severe cases may lead to delirium, coma and death after five to seven days.
Taxonomy and naming
The fungus was first described in 1800, by mycologist Christian Hendrik Persoon, as Helvella esculenta,[4] and gained its current accepted binomial name when the Swedish mycologist Elias Magnus Fries placed it in the genus Gyromitra in 1849.[5] The genus name is derived from the Greek terms gyros/γυρος "round" and mitra/μιτρα "headband".[6] Its specific epithet is derived from the Latin esculentus, "edible".[7]
It is known by a variety of common descriptive names such as "brain mushroom,"[8] "turban fungus,"[9] elephant ears,[10] or "beefsteak mushroom/morel," although beefsteak mushroom can also refer to the much less toxic Fistulina hepatica.[11] Dating from the 19th century, the German term lorchel is a result of the older lorche, itself from the 18th century Low German Lorken, aligning with the similar-sounding (and similar-looking) morchel.[12][13]
Gyromitra esculenta is a member of a group of fungi known as "false morels", so named for their resemblance to the highly regarded true morels of the genus Morchella. The grouping includes other species of the genus Gyromitra, such as G. infula (elfin saddle), G. caroliniana and G. gigas (snow morel). While some of these species contain little to no gyromitrin, many guidebooks recommend treating them all as poisonous, since their similar appearance and significant intraspecific variation can make reliable identification difficult.[14]
The more distantly related ascomycete mushrooms of the genus Verpa, such as V. bohemica and V. conica, are also known as false morels, early morels or thimble morels; like the Gyromitra, they are eaten by some and considered poisonous by others.[15]
The genus Gyromitra had been classically considered part of the family Helvellaceae, along with the similar-looking elfin saddles of the genus Helvella. Analysis of the ribosomal DNA of many of the Pezizales showed Gyromitra esculenta and the other false morels to be only distantly related to the other members of the Helvellaceae and instead most closely related to the genus Discina, forming a clade which also contains Pseudorhizina and Hydnotrya. Thus the four genera are now included in the family Discinaceae.[16]
Description

Resembling a brain, the irregularly shaped cap may be up to 10 centimetres (3.9 in) high and 15 centimetres (5.9 in) wide. Initially smooth, it becomes progressively more wrinkled as it grows and ages. The cap colour may be various shades of reddish-, chestnut-, purplish-, bay-, dark or sometimes golden-brown. Specimens from California may have more reddish-brown caps.[8] Attached to the cap at several points, the stipe is 3–6 centimetres (1.2–2.4 in) high and 2–3 centimetres (0.8–1.2 in) wide. Gyromitra esculenta has a solid stipe whereas those of true morels (Morchella spp.) are hollow.[17] The smell can be pleasant and has been described as fruity, and the fungus is mild-tasting. The spore print is whitish, with transparent spores that are elliptical and 17–22 μm in length.[18]
Gyromitra esculenta resembles the various species of true morel, although the latter are more symmetric and look more like pitted gray, tan, or brown sponges. Its cap is generally darker and larger.[19]
Distribution and habitat
Gyromitra esculenta grows on sandy soil in Temperate coniferous forest and occasionally in deciduous woodlands. Among conifers it is mostly found under pines (Pinus spp.), but also sometimes under aspen (Populus spp.).[20] The hunting period is from April to July, earlier than for other species, and the fungus may even sprout up with the melting snow.[8] It can be abundant in some years and rare in others. The mushroom is more commonly found in places where ground has been disturbed, such as openings, rivulets, washes, timber clearings, plowed openings, forest fire clearings, and roadsides.[17] Enthusiasts in Finland have been reported burying newspaper inoculated with the fungus in the ground in autumn and returning the following spring to collect mushrooms.[21]
Although more abundant in montane and northern coniferous woodlands such as the Sierra Nevada and the Cascade Range in northwestern North America, Gyromitra esculenta is found widely across the continent,[8] as far south as Mexico.[22] It is also common in Central Europe, less abundant in the east, and more in montane areas than lowlands.[9] It has been recorded from Northern Ireland,[23] from Uşak Province in Western Turkey,[24] and from the vicinity of Kaş in the Antalya Province of Turkey's southern coast.[25]
Toxicity

Toxic reactions have been known for at least a hundred years. Experts speculated the reaction was more of an allergic one specific to the consumer, or a misidentification, rather than innate toxicity of the fungus, due to the wide range in effects seen. Some would suffer severely or perish while others exhibited no symptoms after eating similar amounts of mushrooms from the same dish. Yet others would be poisoned after eating Gyromitra esculenta for many years without ill-effects.[26] However, the fungus is now widely recognized as potentially deadly.[27]
Gyromitra esculenta contains levels of the poison gyromitrin that vary locally among populations; although these mushrooms are only rarely involved in poisonings in either North America or western Europe, intoxications are seen frequently in eastern Europe and Scandinavia.[28] A 1971 Polish study reported at the time that the species accounted for up to 23% of mushroom fatalities each year.[29] Death rates have dropped since the mid-twentieth century; in Sweden poisoning is common, though life-threatening poisonings have not been detected and there was no fatality reported over the 50 years from 1952 to 2002.[30] Gyromitra poisonings are rare in Spain, due to the widespread practice of drying the mushrooms before preparation and consumption,[31] but has a mortality rate of about 25%.[32]
A lethal dose of gyromitrin has been estimated to be 10–30 mg/kg for children and 20–50 mg/kg in adults. These doses correspond to around 0.2–0.6 kilograms (0.4–1.3 lb) and 0.4–1 kilogram (0.9–2.2 lb) of fresh mushroom respectively.[33] However, individual responses may vary and people who have ingested similar amounts may develop anything from minimal to severe toxicity.[34] Evidence suggests that children are more severely affected; it is unclear whether this is due to a larger weight consumed per body mass ratio or to differences in enzyme and metabolic activity.[35] Although the amount of gyromitrin present can be significantly reduced through parboiling, there is evidence that repeated consumption can increase risk of toxicity.[34]
Geographical variation
Populations of Gyromitra esculenta appear to vary geographically in their toxicity. A French study has shown that mushrooms collected at higher altitudes have lower concentrations of toxin than those from lower elevations,[35] and there is some evidence that fungi west of the Rocky Mountains in North America contain less toxin than those to the east.[36] However, poisonings in the west have been reported,[37] although less frequently than in Europe.[38]
Biochemistry
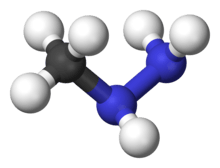
The identity of the toxic constituents eluded researchers until 1968, when acetaldehyde N-methyl-N-formylhydrazone, better known as gyromitrin, was isolated.[39] Gyromitrin is a volatile water-soluble hydrazine compound hydrolyzed in the body into monomethylhydrazine (MMH). Other N-methyl-N-formylhydrazone derivatives have been isolated in subsequent research, although they are present in smaller amounts. These other compounds would also produce monomethylhydrazine when hydrolyzed, although it remains unclear how much each contributes to the false morel's toxicity.[40]
The toxins react with pyridoxal-5-phosphate—the activated form of pyridoxine—and form a hydrazone. This reduces production of the neurotransmitter GABA via decreased activity of glutamic acid decarboxylase,[41] producing the neurological symptoms. MMH also causes oxidative stress leading to methemoglobinemia.[33] Additionally during the metabolism of MMH, N-methyl-N-formylhydrazine is produced; this then undergoes cytochrome P450 regulated oxidative metabolism which via reactive nitrosamide intermediates leads to formation of methyl radicals which lead to liver necrosis.[42][43] Inhibition of diamine oxidase (histaminase) elevates histamine levels resulting in headaches, nausea, vomiting, and abdominal pain.[44]
Symptoms
The symptoms of poisoning are typically gastrointestinal and neurological.[30] Symptoms occur within 6–12 hours of consumption, although cases of more severe poisoning may present sooner—as little as 2 hours after ingestion. Initial symptoms are gastrointestinal, with sudden onset of nausea, vomiting, and watery diarrhea which may be bloodstained. Dehydration may develop if the vomiting or diarrhea is severe. Dizziness, lethargy, vertigo, tremor, ataxia, nystagmus, and headaches develop soon after;[30] fever often occurs, a distinctive feature which does not develop after poisoning by other types of mushrooms.[45] In most cases of poisoning, symptoms do not progress from these initial symptoms, and patients recover after 2–6 days of illness.[29]
In some cases there may be an asymptomatic phase following the initial symptoms which is then followed by more significant toxicity including kidney damage,[46] liver damage, and neurological dysfunction including seizures and coma.[33] These signs usually develop within 1–3 days in serious cases.[30] The patient develops jaundice and the liver and spleen become enlarged, in some cases blood sugar levels will rise (hyperglycemia) and then fall (hypoglycemia) and liver toxicity is seen. Additionally intravascular hemolysis causes destruction of red blood cells resulting in increase in free hemoglobin and hemoglobinuria which can lead to renal toxicity or renal failure. Methemoglobinemia may also occur in some cases. This is where higher than normal levels of methemoglobin, which is a form of hemoglobin that can not carry oxygen, are found in the blood. It causes the patient to become short of breath and cyanotic.[47] Cases of severe poisoning may progress to a terminal neurological phase, with delirium, muscle fasciculations and seizures, and mydriasis progressing to coma, circulatory collapse, and respiratory arrest.[48] Death may occur from five to seven days after consumption.[49]
Treatment
Treatment is mainly supportive; gastric decontamination with activated charcoal may be beneficial if medical attention is sought within a few hours of consumption. However, symptoms often take longer than this to develop, and patients do not usually present for treatment until many hours after ingestion, thus limiting its effectiveness.[50] Patients with severe vomiting or diarrhea can be rehydrated with intravenous fluids.[29] Monitoring of biochemical parameters such as methemoglobin levels, electrolytes, liver and kidney function, urinalysis, and complete blood count is undertaken and any abnormalities are corrected. Dialysis can be used if kidney function is impaired or the kidneys are failing. Hemolysis may require a blood transfusion to replace the lost red blood cells, while methemoglobinemia is treated with intravenous methylene blue.[51]
Pyridoxine, also known as vitamin B6, can be used to counteract the inhibition by MMH on the pyridoxine-dependent step in the synthesis of the neurotransmitter GABA. Thus GABA synthesis can continue and symptoms are relieved.[52] Pyridoxine, which is only useful for the neurological symptoms and does not decrease hepatic toxicity,[43][53] is given at a dose of 25 mg/kg; this can be repeated up to a maximum total of 15 to 30 g daily if symptoms do not improve.[54] Benzodiazepines are given to control seizures; as they also modulate GABA receptors they may potentially increase the effect of pyridoxine. Additionally MMH inhibits the chemical transformation of folic acid into its active form, folinic acid, this can be treated by folinic acid given at 20–200 mg daily.[33]
Carcinogenicity
Monomethylhydrazine,[55] as well as its precursors methylformylhydrazine[56][57] and gyromitrin[58] and raw Gyromitra esculenta,[59] have been shown to be carcinogenic in experimental animals. Although Gyromitra esculenta has not been observed to cause cancer in humans,[60] it is possible there is a carcinogenic risk for people who ingest these types of mushrooms.[56] The toxins may be cumulative[34] and even small amounts may have a carcinogenic effect.[61] At least 11 different hydrazines have been isolated from Gyromitra esculenta, and it is not known if the potential carcinogens can be completely removed by parboiling.[3]
Consumption

Despite its recognized toxicity, Gyromitra esculenta is marketed and consumed in several countries or states in Europe and North America. It was previously consumed in Germany, with fungi picked in and exported from Poland; more recently, however, Germany and Switzerland discouraged consumption by prohibiting its sale.[18][60] Similarly in Sweden, the Swedish National Food Administration warns it is not fit for human consumption,[62] and restricts purchase of fresh mushrooms to restaurants alone.[63] The mushroom is still highly regarded and consumed in Bulgaria, being sold in markets and picked for export there.[64] In some countries such as Spain, especially in the eastern Pyrenees, they are traditionally considered a delicacy, and many people report consuming them for many years with no ill effects.[65] Despite this, the false morel is listed as hazardous in official mushroom lists published by the Catalan Government[14] and sale to the public is prohibited throughout Spain.[66] Outside of Europe, Gyromitra esculenta is consumed in the Great Lakes region and some western states in the United States.[67]
Selling and purchasing fresh false morels is legal in Finland, where it is highly regarded.[68] However, the mushrooms are required by law to be accompanied with a warning that they are poisonous and legally prescribed preparation instructions.[69] False morels are also sold prepared and canned, in which case they are ready to be used. Official figures from the Finnish Ministry of Agriculture and Forestry report a total amount of false morels sold in Finland of 21.9 tonnes in 2006 and 32.7 tonnes, noted as being above average, in 2007.[70] In 2002, the Finnish Food Safety Authority estimated annual consumption of false morels to be hundreds of tonnes in plentiful years.[71] In Finnish cuisine, false morels may be cooked in an omelette, or gently sautéed in butter in a saucepan, flour and milk added to make a bechamel sauce, or pie filling. Alternatively, more fluid can be added for a false morel soup. Typical condiments added for flavour include parsley, chives, dill and black pepper.[72][73]
Controversies
In 2015, Swedish chef Paul Svensson caused a controversy when he prepared a dish with Gyromitra esculenta in a TV show. Mushroom expert Monica Svensson criticized him for including it, because of the mushroom's carcinogenic substances and the risk that inexperienced people might misinterpret the recipe and omit the steps that reduce the toxicity level. She also expressed criticism to Per Morberg for similar reasons. Paul Svensson said that he wasn't aware of the carcinogenic effects and apologized afterwards, and he promised to remove the mushroom from his dishes.[74]
Preparation

Most of the gyromitrin must be removed to render false morels edible. The recommended procedure involves either first drying and then boiling the mushrooms, or boiling the fresh mushrooms directly.[75] To prepare fresh mushroom it is recommended that they are cut into small pieces and parboiled twice in copious amounts of water, at least three parts water to one part chopped mushrooms, for at least five minutes, after each boiling the mushroom should be rinsed thoroughly in clean water.[75] Each round of parboiling reduces the gyromitrin contents to a tenth.[76] The gyromitrin is leached into the water where it will remain, therefore the parboiling water must be discarded and replaced with fresh water after each round of boiling. Drying the mushrooms can also reduce the concentration of gyromitrin; ten days of open air desiccation leads to the loss of 90% of gyromitrin.[34] However it is still recommended that the mushroom be boiled after drying.[75]
MMH boils at 87.5 °C (190 °F) and thus readily vaporizes into the air when water containing fresh false morels is boiled.[37] Poorly ventilated spaces allow vapor to accumulate, resulting in gyromitrin poisoning. If boiling the mushrooms indoors, care should be taken to ensure adequate ventilation, and, if symptoms of gyromitrin poisoning appear, immediately seek fresh air.[77] Even after boiling, small amounts of gyromitrin remain in the mushrooms. Given the possibility of accumulation of toxins, repeated consumption is not recommended.[78]
Prospects for cultivation
Strains with much lower concentrations of gyromitrin have been discovered, and the fungus has been successfully grown to fruiting in culture.[79] Thus there is scope for future research into cultivation of safer strains.[80]
See also
References
- General
- Benjamin, Denis R. (1995). Mushrooms: poisons and panaceas—a handbook for naturalists, mycologists and physicians. New York: WH Freeman and Company. ISBN 0-7167-2600-9.
- Specific
- ↑ "GSD Species Synonymy: Gyromitra esculenta (Pers.) Fr.". Species Fungorum. CAB International. Retrieved 2015-09-29.
- ↑ "Gyromitra". Merriam-Webster Dictionary., "esculent". Merriam-Webster Dictionary..
- 1 2 Dart, Richard C. (2004). "Mushrooms". Medical toxicology. Philadelphia: Williams & Wilkins. pp. 1719–35. ISBN 0-7817-2845-2.
- ↑ Persoon CH (1800) Comm. Schaeff. Icon. Pict.: 64
- ↑ Fries EM (1849) Summa veg. Scand., Section Post. (Stockholm):p. 346
- ↑ Liddell, Henry George and Robert Scott (1980). A Greek–English Lexicon (Abridged ed.). Oxford: Oxford University Press. ISBN 0-19-910207-4.
- ↑ Simpson, D.P. (1979). Cassell's Latin Dictionary (5th ed.). London: Cassell. p. 883. ISBN 0-304-52257-0.
- 1 2 3 4 Arora, David (1986). Mushrooms Demystified: a comprehensive guide to the fleshy fungi (2nd ed.). Berkeley: Ten Speed Press. pp. 801–02. ISBN 0-89815-169-4.
- 1 2 Lamaison, Jean-Louis; Polese, Jean-Marie (2005). The Great Encyclopedia of Mushrooms. Könemann. p. 230. ISBN 3-8331-1239-5.
- ↑ Dearness, J (1924). "Gyromitra poisoning". Mycologia. Mycological Society of America. 16 (4): 199. doi:10.2307/3753381. JSTOR 3753381.
- ↑ Ammirati, Joseph F.; Traquair, James A; Horgen, Paul A (1985). Poisonous mushrooms of the northern United States and Canada. Minneapolis: University of Minnesota Press. p. 122. ISBN 0-8166-1407-5.
- ↑ Dudenredaktion, Bibliographisches Institut, Mannheim (2001). Duden 07 – Das Herkunftswörterbuch – Etymologie der deutschen Sprache (in German). Dudenverlag. ISBN 3-411-04074-2.
- ↑ North, Pamela (1967). Poisonous Plants and Fungi in colour. Blandford Press & Pharmacological Society of Great Britain. p. 109. OCLC 955264.
- 1 2 Departament de Salut, Generalitat de Catalunya. "Bolets" (in Catalan). Retrieved 20 March 2009.
- ↑ Benjamin, p. 267
- ↑ O'Donnell, Kerry; Cigelnik, Elizabeth; Weber, Nancy S.; Trappe, James M. (1997). "Phylogenetic relationships among ascomycetous truffles and the true and false morels inferred from 18S and 28S ribosomal DNA sequence analysis". Mycologia. Mycological Society of America. 89 (1): 48–65. doi:10.2307/3761172. JSTOR 3761172.
- 1 2 Nilsson S, Persson O.(1977) Fungi of Northern Europe 1: Larger Fungi (Excluding Gill Fungi). pp. 34–35. Penguin Books. ISBN 0-14-063005-8
- 1 2 Zeitlmayr, Linus (1976). Wild Mushrooms:An Illustrated Handbook. Hertfordshire: Garden City Press. p. 112. ISBN 0-584-10324-7.
- ↑ Ammirati, Joseph F. p. 121
- ↑ Smith HV, Smith AH (1973). How to Know the Non-Gilled Fleshy Fungi. Dubuque, Il: Wm. C. Brown Co. ISBN 0-697-04866-7.
- ↑ Kuo M (January 2005). "Gyromitra esculenta". MushroomExpert.Com Web site. self. Retrieved 11 May 2008.
- ↑ Medel, Rosario (2005). "A review of the genus Gyromitra (Ascomycota, Pezizales, Discinaceae) in Mexico". Mycotaxon. 94: 103–10.
- ↑ "Northern Ireland's Herbarium Specimens". Northern Ireland Fungus Group. 2007. Retrieved 6 March 2008.
- ↑ Türkoglu A, Alli H, Iṣiloğlu M, Yağiz D, Gezer K (February 2008). "Macrofungal diversity of Uşak province in Turkey" (PDF). Mycotaxon. 103: 1–11. Retrieved 7 March 2008.
- ↑ Gezer K (2000). "Contributions to the Macrofungi Flora of Antalya Province". Turkish Journal of Botany. 24 (5): 293–98. Retrieved 16 February 2008.
- ↑ Benjamin, p. 264
- ↑ Benjamin, p. 265
- ↑ Diaz JH (2005). "Syndromic diagnosis and management of confirmed mushroom poisonings". Critical Care Medicine. 33 (2): 427–36. doi:10.1097/01.CCM.0000153531.69448.49. PMID 15699849.
- 1 2 3 Lampe KF (1979). "Toxic fungi". Annual Review of Pharmacology and Toxicology. 19 (1): 85–104. doi:10.1146/annurev.pa.19.040179.000505. PMID 378111.
- 1 2 3 4 Karlson-Stiber C, Persson H (2003). "Cytotoxic fungi—an overview". Toxicon. 42 (4): 339–49. doi:10.1016/S0041-0101(03)00238-1. PMID 14505933.
- ↑ Palomar Martínez, M.; Piqueras Carrasco, J. (1999). "Intoxicaciones por setas (micetismos)". Retrieved 20 March 2009.
- ↑ Lloret i Carbó, Josep (2004). Protocolos terapéuticos de urgencias (4ª ed.). Elsevier. p. 649. ISBN 84-458-1418-4.
- 1 2 3 4 Michelot D, Toth B (1991). "Poisoning by Gyromitra esculenta—a review". Journal of applied toxicology. 11 (4): 235–43. doi:10.1002/jat.2550110403. PMID 1939997.
- 1 2 3 4 Coulet M, Guillot J (1982). "Poisoning by Gyromitra : a possible mechanism". Medical Hypotheses. 8 (4): 325–34. doi:10.1016/0306-9877(82)90024-X. PMID 7099057.
- 1 2 Benjamin, p. 272
- ↑ Benjamin, p. 140
- 1 2 Leathem AM, Dorran TJ (2007). "Poisoning due to raw Gyromitra esculenta (false morels) west of the Rockies". Canadian Journal of Emergency Medical Care. 9 (2): 127–30. doi:10.1017/s1481803500014937. PMID 17391587.
- ↑ Balterowich L, Blaney B, White S (1996). "Acute hepatotoxicity following ingestion of Gyromitra esculenta(false morel) mushrooms" (pdf). Journal of toxicology. Clinical toxicology. 34 (5): 602.
- ↑ List PH, Luft P (1968). "[Gyromitrin, the poison of Gyromitra esculenta. 16. On the fungi contents]". Archiv der Pharmazie und Berichte der Deutschen Pharmazeutischen Gesellschaft (in German). 301 (4): 294–305. doi:10.1002/ardp.19683010410. PMID 5244383.
- ↑ Pyysalo H (1975). "Some new toxic compounds in false morels, Gyromitra esculenta". Naturwissenschaften. 62 (8): 395. doi:10.1007/BF00625355. PMID 1238907.
- ↑ Cornish HH (1969). "The role of vitamin B6 in the toxicity of hydrazines". Annals of the New York Academy of Sciences. 166 (1): 136–45. doi:10.1111/j.1749-6632.1969.tb54264.x. PMID 5262010.
- ↑ Braun R, Greeff U, Netter KJ (1980). "Indications for nitrosamide formation from the mushroom poison gyromitrin by rat liver microsomes". Xenobiotica. 10 (7–8): 557–64. doi:10.3109/00498258009033790. PMID 7445522.
- 1 2 Braun R, Greeff U, Netter KJ (1979). "Liver injury by the false morel poison gyromitrin". Toxicology. 12 (2): 155–63. doi:10.1016/0300-483X(79)90042-8. PMID 473232.
- ↑ Biegański T, Braun R, Kusche J (1984). "N-methyl-N-formylhydrazine: a toxic and mutagenic inhibitor of the intestinal diamine oxidase". Agents and Actions. 14 (3-4): 351–5. doi:10.1007/BF01973825. PMID 6428190.
- ↑ Benjamin, p. 273
- ↑ Braun R, Kremer J, Rau H (1979). "Renal functional response to the mushroom poison gyromitrin". Toxicology. 13 (2): 187–96. doi:10.1016/s0300-483x(79)80022-0. PMID 42171.
- ↑ Benjamin, p. 274
- ↑ Giusti GV, Carnevale A (1974). "A case of fatal poisoning by Gyromitra esculenta". Archives of toxicology. 33 (1): 49–54. PMID 4480349.
- ↑ Hanrahan JP, Gordon MA (1984). "Mushroom poisoning. Case reports and a review of therapy". JAMA. 251 (8): 1057–61. doi:10.1001/jama.251.8.1057. PMID 6420582.
- ↑ Köppel C (1993). "Clinical symptomatology and management of mushroom poisoning". Toxicon. 31 (12): 1513–40. doi:10.1016/0041-0101(93)90337-I. PMID 8146866.
- ↑ Benjamin, p. 276
- ↑ Wright AV, Niskanen A, Pyysalo H, Korpela H (1981). "Amelioration of toxic effects of ethylidene gyromitrin (false morel poison) with pyridoxine chloride". Journal of Food Safety. 3 (3): 199–203. doi:10.1111/j.1745-4565.1981.tb00422.x.
- ↑ Toth B, Erickson J (1977). "Reversal of the toxicity of hydrazine an analogues by pyridoxine hydrochloride". Toxicology. 7 (1): 31–36. doi:10.1016/0300-483X(77)90035-X. PMID 841582.
- ↑ Kirklin JK, Watson M, Bondoc CC, Burke JF (1976). "Treatment of hydrazine-induced coma with pyridoxine". New England Journal of Medicine. 294 (17): 938–9. doi:10.1056/NEJM197604222941708. PMID 815813.
- ↑ Toth B, Shimizu H (1973). "Methylhydrazine tumorigenesis in Syrian golden hamsters and the morphology of malignant histiocytomas". Cancer Research. 33 (11): 2744–53. PMID 4355982.
- 1 2 Toth B, Nagel D (1978). "Tumors induced in mice by N-methyl-N-formylhydrazine of the false morel Gyromitra esculenta". Journal of the National Cancer Institute. 60 (1): 201–04. PMID 628017.
- ↑ Toth B, Patil K, Erickson J, Kupper R (1979). "False morel mushroom Gyromitra esculenta toxin: N-methyl-N-formylhdrazine carcinogenesis in mice". Mycopathologia. 68 (2): 121–28. doi:10.1007/BF00441091. PMID 573857.
- ↑ Toth B, Smith JW, Patil KD (1981). "Cancer induction in mice with acetaldehyde methylformylhydrazone of the false morel mushroom". Journal of the National Cancer Institute. 67 (4): 881–87. PMID 6944556.
- ↑ Toth B, Patil K, Pyysalo H, Stessman C, Gannett P (1992). "Cancer induction in mice by feeding the raw false morel mushroom Gyromitra esculenta". Cancer Research. 52 (8): 2279–84. PMID 1559231.
- 1 2 Bresinsky A, Besl H (1990). A Colour Atlas of Poisonous Fungi. Wolfe Publishing. pp. 62–68. ISBN 0-7234-1576-5.
- ↑ Benjamin, p. 128–29
- ↑ Andersson, Christer (2007). "Stenmurklan – olämplig att äta". Livsmedelsverket (National Food Administration) (in Swedish). Swedish National Food Administration. Retrieved 7 March 2008.
- ↑ Andersson, Christer (2007). "Stenmurkla - frågor och svar". Livsmedelsverket (National Food Administration) (in Swedish). Swedish National Food Administration. Retrieved 4 March 2012.
- ↑ Drumeva-Dimcheva M, Gyosheva-Bogoeva M (1998). "Section One: Bulgaria's Biological Diversity – The Macromycetes Fungi of Bulgaria". Bulgaria's Biological Diversity: Conservation Status and Needs Assessment. Biodiversity Support Program (WWF, The Nature Conservancy, and World Resources Institute Consortium). Retrieved 2008-03-06.
- ↑ "Bolets". Revista el cargol. 2008. Retrieved 8 June 2008.
- ↑ Ministerio de Sanidad y Consumo (6 February 2004). "ORDEN SCO/190/2004, de 28 de enero, por la que se establece la lista de plantas cuya venta al público queda prohibida o restringida por razón de su toxicidad" (PDF). BOE (in Spanish) (32): 5061–65. Retrieved 8 June 2008.
- ↑ Simons, DM (1971). "The Mushroom Toxins". Delaware Medical Journal. 43: 177–87.
- ↑ Härkönen, M (1998). "Uses of mushrooms by Finns and Karelians". International Journal of circumpolar Health. 57 (1): 40–55. PMID 9567575.
- ↑ "False morels must be accompanied by warning and handling instructions". The Finnish Food Safety Authority Evira. 11 May 2006. Retrieved 2008-03-04.
- ↑ Suomen Gallup Elintarviketieto Oy (March 2007). MARSI 2007 – Luonnonmarjojen ja -sienien kauppaantulomäärät vuonna 2007 [Amounts of wild berries and mushrooms offered for sale in 2007] (in Finnish). Helsinki: Finnish Ministry of Agriculture and Forestry. p. 10.
- ↑ Finnish Food Safety Authority (2002). Riskiraportti – elintarvikkeiden ja Talousveden kemialliset vaarat [Risk report on toxins in food and tapwater] (in Finnish). p. 38.
- ↑ "Kevät on aikaa korvasienen ja väinönputken" (in Finnish). Lapin Keittiömestarit. Archived from the original on 26 May 2008. Retrieved 22 June 2008.
- ↑ Davidson A (2003). North Atlantic Seafood: A Comprehensive Guide with Recipes. Ten Speed Press. p. 361. ISBN 1-58008-450-8.
- ↑ Nilsson, Kerstin (26 April 2015). "Här lagar tv-kocken Paul Svensson mat med giftsvamp" (in Swedish). Aftonbladet. Retrieved 26 April 2015.
- 1 2 3 "False Morel Fungi – poisonous when raw". The Finnish Food Safety Authority Evira. 2008. Archived from the original on 18 December 2007. Retrieved 2008-03-04.
- ↑ Pyysalo H, Niskanen A (1977). "On the occurrence of N-methyl-N-formylhydrazones in fresh and processed false morel, Gyromitra esculenta". Journal of Agricultural and Food Chemistry. 25 (3): 644–47. doi:10.1021/jf60211a006. PMID 558239.
- ↑ Benjamin, p. 269
- ↑ Benjamin, p. 278
- ↑ List PH, Sundermann G (1974). "Achtung! Frühjahrslorcheln". Deutsche Apotheker Zeitung. 114: 331–32.
- ↑ Benjamin, p. 279
External links
| Wikimedia Commons has media related to Gyromitra esculenta. |
- "Gyromitra esculenta, one of the false morels"
- California Fungi—Gyromitra esculenta
- Official Finnish instructions for the processing of false morels