Gut flora
Gut flora (gut microbiota, or gastrointestinal microbiota) is the complex community of microorganisms that live in the digestive tracts of humans and other animals, including insects. The gut metagenome is the aggregate of all the genomes of gut microbiota.[1] The gut is one niche that human microbiota inhabit.[2]
In humans, the gut microbiota has the largest numbers of bacteria and the greatest number of species compared to other areas of the body.[3] In humans the gut flora is established at one to two years after birth, and by that time the intestinal epithelium and the intestinal mucosal barrier that it secretes have co-developed in a way that is tolerant to, and even supportive of, the gut flora and that also provides a barrier to pathogenic organisms.[4][5]
The relationship between some gut flora and humans is not merely commensal (a non-harmful coexistence), but rather a mutualistic relationship.[2]:700 Some human gut microorganisms benefit the host by fermenting dietary fiber into short-chain fatty acids (SCFAs), such as acetic acid and butyric acid, which are then absorbed by the host.[3][6] Intestinal bacteria also play a role in synthesizing vitamin B and vitamin K as well as metabolizing bile acids, sterols, and xenobiotics.[2][6] The systemic importance of the SCFAs and other compounds they produce are like hormones and the gut flora itself appears to function like an endocrine organ,[6] and dysregulation of the gut flora has been correlated with a host of inflammatory and autoimmune conditions.[3][7]
The composition of human gut flora changes over time, when the diet changes, and as overall health changes.[3][7] A systematic review from 2016 examined the preclinical and small human trials that have been conducted with certain commercially available strains of probiotic bacteria and identified those that had the most potential to be useful for certain central nervous system disorders.[8]
Types
The microbial composition of the gut flora varies across the digestive tract. In the stomach and small intestine, relatively few species of bacteria are generally present.[9][10] The colon, in contrast, contains a densely-populated microbial ecosystem with up to 1012 cells per gram of intestinal content.[9] These bacteria represent between 300 and 1000 different species.[9][10] However, 99% of the bacteria come from about 30 or 40 species.[11] As a consequence of their abundance in the intestine, bacteria also make up to 60% of the dry mass of feces.[12] Fungi, archaea, and viruses are also present in the gut flora, but less is known about their activities.[13]
Over 99% of the bacteria in the gut are anaerobes, but in the cecum, aerobic bacteria reach high densities.[2] It is estimated that these gut flora have around a hundred times as many genes in total as there are in the human genome.[14]
Many species in the gut have not been studied outside of their hosts because most cannot be cultured.[10][11][15] While there are a small number of core species of microbes shared by most individuals, populations of microbes can vary widely among different individuals.[16] Within an individual, microbe populations stay fairly constant over time, even though some alterations may occur with changes in lifestyle, diet and age.[9][17] The Human microbiome project has set out to better describe the microflora of the human gut and other body locations.
The four dominant bacterial phyla in the human gut are Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria.[18] Most bacteria belong to the genera Bacteroides, Clostridium, Faecalibacterium,[9][11] Eubacterium, Ruminococcus, Peptococcus, Peptostreptococcus, and Bifidobacterium.[9][11] Other genera, such as Escherichia and Lactobacillus, are present to a lesser extent.[9] Species from the genus Bacteroides alone constitute about 30% of all bacteria in the gut, suggesting that this genus is especially important in the functioning of the host.[10]
Fungal genera that have been detected in the gut include Candida, Saccharomyces, Aspergillus, Penicillium, Rhodotorula, Trametes, Pleospora, Sclerotinia, Bullera, and Galactomyces, among others.[19][20] Rhodotorula is most frequently found in individuals with inflammatory bowel disease while Candida is most frequently found in individuals with hepatitis B cirrhosis and chronic hepatitis B.[19]
Archaea constitute another large class of gut flora which are important in the metabolism of the bacterial products of fermentation.
Enterotype
An enterotype is a classification of living organisms based on its bacteriological ecosystem in the human gut microbiome not dictated by age, gender, body weight, or national divisions.[21] There are indications that long-term diet influences enterotype.[22] Three human enterotypes have been discovered.[21][23]
Flora composition
Anatomy
Stomach flora
Due to the high acidity of the stomach, most microorganisms cannot survive. The main bacterial inhabitants of the stomach include: Streptococcus, Staphylococcus, Lactobacillus, Peptostreptococcus, and types of yeast.[2]:720 Helicobacter pylori is a Gram-negative spiral organism that establishes on gastric mucosa causing chronic gastritis and peptic ulcer disease and is a carcinogen for gastric cancer.[2]:904
Intestinal flora
| Bacteria commonly found in the human colon[24] | |
| Bacterium | Incidence (%) |
|---|---|
| Bacteroides fragilis | 100 |
| Bacteroides melaninogenicus | 100 |
| Bacteroides oralis | 100 |
| Enterococcus faecalis | 100 |
| Escherichia coli | 100 |
| Enterobacter sp. | 40–80 |
| Klebsiella sp. | 40–80 |
| Bifidobacterium bifidum | 30–70 |
| Staphylococcus aureus | 30–50 |
| Lactobacillus | 20–60 |
| Clostridium perfringens | 25–35 |
| Proteus mirabilis | 5–55 |
| Clostridium tetani | 1–35 |
| Clostridium septicum | 5–25 |
| Pseudomonas aeruginosa | 3–11 |
| Salmonella enteritidis | 3–7 |
| Faecalibacterium prausnitzii | ?common |
| Peptostreptococcus sp. | ?common |
| Peptococcus sp. | ?common |
The small intestine contains a trace amount of microorganisms due to the proximity and influence of the stomach. Gram positive cocci and rod shaped bacteria are the predominant microorganisms found in the small intestine.[2] However, in the distal portion of the small intestine alkaline conditions support gram-positive bacteria of the Enterobacteriaceae.[2] The bacterial flora of the small intestine aid in a wide range of intestinal functions. The bacterial flora provide regulatory signals that enable the development and utility of the gut. Overgrowth of bacteria in the small intestine can lead to intestinal failure.[25] In addition the large intestine contains the largest bacterial ecosystem in the human body.[2] Factors that disrupt the microorganism population of the large intestine include antibiotics, stress, and parasites.[2]
Bacteria make up most of the flora in the colon[26] and 60% of the dry mass of feces.[9] This fact makes feces an ideal source to test for gut flora for any tests and experiments by extracting the nucleic acid from fecal specimens, and bacterial 16S rRNA gene sequences are generated with bacterial primers. This form of testing is also often preferable to more invasive techniques, such as biopsies. Somewhere between 300[9] and 1000 different species live in the gut,[10] with most estimates at about 500.[27][28] However, it is probable that 99% of the bacteria come from about 30 or 40 species, with Faecalibacterium prausnitzii being the most common species in healthy adults.[11][29] Fungi and protozoa also make up a part of the gut flora, but little is known about their activities. The virome is mostly bacteriophages.[30]
Research suggests that the relationship between gut flora and humans is not merely commensal (a non-harmful coexistence), but rather is a mutualistic, symbiotic relationship.[10] Though people can (barely) survive with no gut flora,[27] the microorganisms perform a host of useful functions, such as fermenting unused energy substrates, training the immune system via end products of metabolism like propionate and acetate, preventing growth of harmful species, regulating the development of the gut, producing vitamins for the host (such as biotin and vitamin K), and producing hormones to direct the host to store fats.[2]:713ff Extensive modification and imbalances of the gut microbiota and its microbiome or gene collection are associated with obesity.[31] However, in certain conditions, some species are thought to be capable of causing disease by causing infection or increasing cancer risk for the host.[9][26]
Age
It has been demonstrated that there are common patterns of microbiome composition evolution during life.[32] In general, the diversity of microbiota composition of fecal samples is significantly higher in adults than in children, although interpersonal differences are higher in children than in adults.[33] Much of the maturation of microbiota into an adult-like configuration happens during the three first years of life.[33]
As the microbiome composition changes, so does the composition of bacterial proteins produced in the gut. In adult microbiomes, a high prevalence of enzymes involved in fermentation, methanogenesis and the metabolism of arginine, glutamate, aspartate and lysine have been found. In contrast, in infant microbiomes the dominant enzymes are involved in cysteine metabolism and fermentation pathways.[33]
Diet
Studies and statistical analyses have identified the different bacterial genera in gut microbiota and their associations with nutrient intake. Gut microflora is mainly composed of three enterotypes: Prevotella, Bacteroides, and Ruminococcus. There is an association between the concentration of each microbial community and diet. For example, Prevotella is related to carbohydrates and simple sugars, while Bacteroides is associated with proteins, amino acids, and saturated fats. One enterotype will dominate depending on the diet. Altering the diet will result in a corresponding change in the numbers of species.[22]
Malnourished human children have less mature and less diverse gut microbiota than healthy children, and changes in the microbiome associated with nutrient scarcity can in turn be a pathophysiological cause of malnutrition.[34][35] Malnourished children also typically have more potentially pathogenic gut flora, and more yeast in their mouths and throats.[36]
Geography
Gut microbiome composition depends on the geographic origin of populations. Variations in trade off of Prevotella, the representation of the urease gene, and the representation of genes encoding glutamate synthase/degradation or other enzymes involved in amino acids degradation or vitamin biosynthesis show significant differences between populations from USA, Malawi or Amerindian origin.[33]
The US population has a high representation of enzymes encoding the degradation of glutamine and enzymes involved in vitamin and lipoic acid biosynthesis; whereas Malawi and Amerindian populations have a high representation of enzymes encoding glutamate synthase and they also have an overrepresentation of α-amylase in their microbiomes. As the US population has a diet richer in fats than Amerindian or Malawian populations which have a corn-rich diet, the diet is probably a main determinant of gut bacterial composition.[33]
Further studies have indicated a large difference in the composition of microbiota between European and rural African children. The fecal bacteria of children from Florence were compared to that of children from the small rural village of Boulpon in Burkina Faso. The diet of a typical child living in this village is largely lacking in fats and animal proteins and rich in polysaccharides and plant proteins. The fecal bacteria of European children was dominated by Firmicutes and showed a marked reduction in biodiversity, while the fecal bacteria of the Boulpon children was dominated by Bacteroidetes. The increased biodiversity and different composition of gut flora in African populations may aid in the digestion of normally indigestible plant polysaccharides and also may result in a reduced incidence of non-infectious colonic diseases.[37]
On a smaller scale, it has been shown that sharing numerous common environmental exposures in a family is a strong determinant of individual microbiome composition. This effect has no genetic influence and it is consistently observed in culturally different populations.[33]
Acquisition of gut flora in human infants
In humans, a gut flora similar to an adult's is formed within one to two years of birth.[4] The gastrointestinal tract of a normal fetus has been considered to be sterile, however recently it has been acknowledged that microbial colonisation may occur in the fetus.[38] During birth and rapidly thereafter, bacteria from the mother and the surrounding environment colonize the infant's gut.[4] As of 2013, it was unclear whether most of colonizing arise from the mother or not.[4] Infants born by caesarean section may also be exposed to their mothers' microflora, but the initial exposure is most likely to be from the surrounding environment such as the air, other infants, and the nursing staff, which serve as vectors for transfer.[38] During the first year of life, the composition of the gut flora is generally simple and it changes a great deal with time and is not the same across individuals.[4]
The initial bacterial population are generally facultative anaerobic organisms; investigators believe that these initial colonizers decrease the oxygen concentration in the gut, which in turn allows purely aneorobic bacteria like Bacteroides, Actinobacteria, and Firmicutes to become established and thrive.[4] Breast-fed babies become dominated by bifidobacteria, possibly due to the contents of bifidobacterial growth factors in breast milk.[39][40] In contrast, the microbiota of formula-fed infants is more diverse, with high numbers of Enterobacteriaceae, enterococci, bifidobacteria, Bacteroides, and clostridia.[41]
Functions
When the gut flora first started to be studied, it was thought to have three key roles: directly defending against pathogens, fortifying host defense by its role in developing and maintaining the intestinal epithelium and inducing antibody production there, and metabolizing otherwise undigestable compounds in food; subsequent work discovered its role in training the developing immune system, and yet further work focused on its role in the gut-brain axis.[42]
Direct inhibition of pathogens
The gut flora community plays a direct role in defending against pathogens by fully colonizing the space, making use of all available nutrients, and by secreting compounds that kill or inhibit unwelcome organisms that would compete for nutrients with it.[43] Disruption of the gut flora allows competing organisms like Clostridium difficile to become established that otherwise are kept in abeyance.[43]
Development of enteric protection and immune system

In humans, a gut flora similar to an adult's is formed within one to two years of birth.[4] As the gut flora gets established, the lining of the intestines – the intestinal epithelium and the intestinal mucosal barrier that it secretes – develop as well, in a way that is tolerant to, and even supportive of, commensurate microorganisms to a certain extent and also provides a barrier to pathogenic ones.[4] Specifically, goblet cells that produce the muscosa proliferate, and the mucosa layer thickens, providing an outside mucosal layer in which "friendly" microorganisms can anchor and feed, and an inner layer that even these organisms cannot penetrate.[4][5] Additionally, the development of gut-associated lymphoid tissue (GALT), which forms part of the intestinal epithelium and which detects and reacts to pathogens, appears and develops during the time that the gut flora develops and established.[4] The GALT that develops is tolerant to gut flora species, but not to other microorganisms.[4] GALT also normally becomes tolerant to food to which the infant is exposed, as well as digestive products of food, and gut flora's metabolites produced from food.[4]
The human immune system creates cytokines that can drive the immune system to produce inflammation in order to protect itself, and that can tamp down the immune response to maintain homeostasis and allow healing after insult or injury.[4] Different bacterial species that appear in gut flora have been shown to be able to drive the immune system to create cytokines selectively; for example Bacteroides fragilis and some Clostridia species appear to drive an anti-inflammatory response, while some segmented filamentous bacteria drive the production of inflammatory cytokines.[4][44] Gut flora can also regulate the production of antibodies by the immune system.[4][45] These cytokines and antibodies can have effects outside the gut, in the lungs and other tissues.[4]
Metabolism
Without gut flora, the human body would be unable to utilize some of the undigested carbohydrates it consumes, because some types of gut flora have enzymes that human cells lack for breaking down certain polysaccharides.[6] Rodents raised in a sterile environment and lacking in gut flora need to eat 30% more calories just to remain the same weight as their normal counterparts.[6] Carbohydrates that humans cannot digest without bacterial help include certain starches, fiber, oligosaccharides, and sugars that the body failed to digest and absorb like lactose in the case of lactose intolerance and sugar alcohols, mucus produced by the gut, and proteins.[3][6]
Bacteria turn carbohydrates they ferment into short-chain fatty acids (SCFAs)[11][28] by a form of fermentation called saccharolytic fermentation.[28] Products include acetic acid, propionic acid and butyric acid.[11][28] These materials can be used by host cells, providing a major source of useful energy and nutrients for humans,[28] as well as helping the body to absorb essential dietary minerals such as calcium, magnesium and iron.[9] Gases and organic acids, such as lactic acid, are also produced by saccharolytic fermentation.[11] Acetic acid is used by muscle, propionic acid helps the liver produce ATP, and butyric acid provides energy to gut cells and may prevent cancer.[28] Evidence also indicates that bacteria enhance the absorption and storage of lipids[10] and produce and then facilitate the body to absorb needed vitamins like vitamin K.
Gut flora also synthesize vitamins like biotin and folate, and help with absorption of dietary elements including magnesium, calcium and iron.[17] Methanogenic archae such as Methanobrevibacter smithii are involved in the removal of end products of bacterial fermentation such as hydrogen.[2]
Gut-brain axis
The gut–brain axis is the biochemical signaling that takes place between the gastrointestinal tract and the central nervous system.[42] That term has been expanded to include the role of the gut flora in the interplay; the term "microbiome-gut-brain axis" is sometimes used to describe paradigms explicitly including the gut flora.[42][50][51]
Broadly defined, the gut-brain axis includes the central nervous system, neuroendocrine and neuroimmune systems including the hypothalamic–pituitary–adrenal axis (HPA axis), sympathetic and parasympathetic arms of the autonomic nervous system including the enteric nervous system, the vagus nerve, and the gut microbiota.[42][51]
Interest in the field was sparked by a 2004 study showing that germ-free mice showed an exaggerated HPA axis response to stress compared to non-GF laboratory mice.[42] As of January 2016, most of the work that has been done on the role of gut flora in the gut-brain axis had been conducted in animals, or characterizing the various neuroactive compounds that gut flora can produce, and studies with humans measuring differences between people with various psychiatric and neurological differences, or changes to gut flora in response to stress, or measuring effects of various probiotics (dubbed "psychobiotics in this context), had generally been small and could not be generalized; whether changes to gut flora are a result of disease, a cause of disease, or both in any number of possible feedback loops in the gut-brain axis, remained unclear.[42][52]
A systematic review from 2016 examined the preclinical and small human trials that have been conducted with certain commercially available strains of probiotic bacteria and found that among those tested, Bifidobacterium and Lactobacillus genera (B. longum, B. breve, B. infantis, L. helveticus, L. rhamnosus, L. plantarum, and L. casei), had the most potential to be useful for certain central nervous system disorders.[8]
Alterations in flora balance
Effects of antibiotic use
Altering the numbers of gut bacteria, for example by taking broad-spectrum antibiotics, may affect the host's health and ability to digest food.[53] Antibiotics can cause antibiotic-associated diarrhea (AAD) by irritating the bowel directly, changing the levels of gut flora, or allowing pathogenic bacteria to grow.[11] Another harmful effect of antibiotics is the increase in numbers of antibiotic-resistant bacteria found after their use, which, when they invade the host, cause illnesses that are difficult to treat with antibiotics.[53]
Changing the numbers and species of gut flora can reduce the body's ability to ferment carbohydrates and metabolize bile acids and may cause diarrhea. Carbohydrates that are not broken down may absorb too much water and cause runny stools, or lack of SCFAs produced by gut flora could cause the diarrhea.[11]
A reduction in levels of native bacterial species also disrupts their ability to inhibit the growth of harmful species such as C. difficile and Salmonella kedougou, and these species can get out of hand, though their overgrowth may be incidental and not be the true cause of diarrhea.[9][11][53] Emerging treatment protocols for C. difficile infections involve fecal microbiota transplantation of donor feces. (see Fecal transplant). Initial reports of treatment describe success rates of 90%, with few side effects. Efficacy is speculated to result from restoring bacterial balances of bacteroides and firmicutes classes of bacteria.[54]
Gut flora composition also changes in severe illnesses, due not only to antibiotic use but also to such factors as ischemia of the gut, failure to eat, and immune compromise. Negative effects from this have led to interest in selective digestive tract decontamination (SDD), a treatment to kill only pathogenic bacteria and allow the re-establishment of healthy ones.[55]
Antibiotics alter the population of the gastrointestinal (GI) tract microbiota, may change the intra-community metabolic interactions, modify caloric intake by using carbohydrates, and globally affects host metabolic, hormonal and immune homeostasis.[56]
There is reasonable evidence that taking probiotics containing Lactobacillus species may help prevent antibiotic-associated diarrhea and that taking probiotics with Saccharomyces (e.g., Saccharomyces boulardii ) may help to prevent Clostridium difficile infection following systemic antibiotic treatment.[52]
Pregnancy
Women's gut microbiota change as pregnancy advances, with the changes similar to those seen in metabolic syndromes such as diabetes. The change in gut flora causes no ill effects. The newborn's gut biota resemble the mother's first-trimester samples. The diversity of the flora decreases from the first to third trimester, as the numbers of certain species go up.[57]
Probiotics, prebiotics, synbiotics, and pharmabiotics
Probiotics are microorganisms that are believed to provide health benefits when consumed.[58][59] With regard to gut flora, prebiotics are typically non-digestible, fiber compounds that pass undigested through the upper part of the gastrointestinal tract and stimulate the growth or activity of advantageous gut flora by acting as substrate for them.[28][60]
Synbiotics refers to food ingredients or dietary supplements combining probiotics and prebiotics in a form of synergism.[61]
The term "pharmabiotics" is used in various ways, to mean: pharmaceutical formulations (standardized manufacturing that can obtain regulatory approval as a drug) of probiotics, prebiotics, or synbiotics;[62] probiotics that have been genetically engineered or otherwise optimized for best performance (shelf life, survival in the digestive tract, etc.);[63] and the natural products of gut flora metabolism (vitamins, etc.).[64]
There is some evidence that treatment with some probiotic strains of bacteria may be effective in irritable bowel syndrome and chronic idiopathic constipation. Those organisms most likely to result in a decrease of symptoms have included:
- Streptococcus faecium
- Lactobacillus plantarum
- Lactobacillus rhamnosus
- Propionibacterium freudenreichii
- Bifidobacterium breve
- Lactobacillus reuteri
- Lactobacillus salivarius
- Bifidobacterium infantis
- Streptococcus thermophilus[65][66][67]
Gram positive bacteria present in the lumen may be associated with extending the duration of relapse for ulcerative colitis.[66]
Role in disease
Bacteria in the digestive tract can contribute to and be affected by disease in various ways. The presence or overabundance of some kinds of bacteria may contribute to inflammatory disorders such as inflammatory bowel disease.[9] Additionally, metabolites from certain members of the gut flora may influence host signaling pathways, contributing to disorders such as obesity and colon cancer.[9] Alternatively, in the event of a breakdown of the gut epithelium, the intrusion of gut flora components into other host compartments can lead to sepsis.[9]
Ulcers
Helicobacter pylori can cause stomach ulcers by crossing the epithelial lining of the stomach. Here the body produces an immune response. During this response parietal cells are stimulated and release extra hydrochloric acid (HCl+) into the stomach. However, the response does not stimulate the mucus-secreting cells that protect and line the epithelium of the stomach. The extra acid sears holes into the epithelial lining of the stomach, resulting in stomach ulcers.[32]
Inflammatory bowel diseases
The two main types of inflammatory bowel diseases, Crohn's disease and ulcerative colitis, are chronic inflammatory disorders of the gut; the causes of these disease are unknown and issues with the gut flora and its relationship with the host have been implicated in these conditions.[7][68][69][70] Additionally, it appears that interactions of gut flora with the gut-brain axis have a role in IBD, with physiological stress mediated through the hypothalamic–pituitary–adrenal axis driving changes to intestinal epithelium and the gut flora in turn releasing factors and metabolites that trigger sigalling in the enteric nervous system and the vagus nerve.[1]
The diversity of gut flora appears to be significantly diminished in people with inflammatory bowel diseases compared to healthy people; additionally, in people with ulcerative colitis, Proteobacteria and Actinobacteria appear to dominate; in people with Crohn's, Enterococcus faecium and several Proteobacteria appear to be over-represented.[1]
There is reasonable evidence that correcting gut flora imbalances by taking probiotics with Lactobacilli and Bifidobacteria can reduce visceral pain and gut inflammation in IBD.[52]
Irritable bowel syndrome
Irritable bowel syndrome is a result of stress and chronic activation of the HPA axis; its symptoms include abdominal pain, changes in bowel movements, and an increase in proinflammatory cytokines. Overall, studies have found that the luminal and mucosal microbiota are changed in irritable bowel syndrome individuals, and these changes can relate to the type of irritation such as diarrhea or constipation. Also, there is a decrease in the diversity of the microbiome with low levels of fecal Lactobacilli and Bifidobacteria, high levels of facultative anaerobic bacteria such as Escherichia coli, and increased ratios of Firmicutes:Bacteroidetes.[51]
Other inflammatory or autoimmune conditions
Allergy, asthma and Diabetes mellitus type 1 are autoimmune and inflammatory disorders' the causes of these disease are unknown and issues with the gut flora and its relationship with the host have been implicated in these conditions.[7]
Two hypotheses have been posed to explain the rising prevalence of these diseases in the developed world: the hygiene hypothesis, which posits that children in the developed world are not exposed to a wide enough range of pathogens and end up with an overreactive immune system, and the role of the Western pattern diet which lacks whole grains and fiber and has an overabundance of simple sugars.[7] Both hypotheses converge on the changes in the gut flora and its role in modulating the immune system, and as of 2016 this was an active area of research.[7]
Similar hypotheses have been posited for the rise of food and other allergies.[71]
As of 2016 it was not clear if changes to the gut flora cause these auto-immune and inflammatory disorders or are a product of them or adaptation to them.[7][72]
Obesity and metabolic syndrome
The gut flora has also been implicated in obesity and metabolic syndrome due to the key role it plays in the digestive process; the Western pattern diet appears to drive and maintain changes in the gut flora that in turn change how much energy is derived from food and how that energy is used.[70][73] One aspect of a healthy diet that is often lacking in the Western-pattern diet is fiber and other complex carbohydrates that a healthy gut flora require to flourish; changes to gut flora in response to a Western-pattern diet appear to increase the amount of energy generated by the gut flora which may contribute to obesity and metabolic syndrome.[52] There is also evidence that microbiota influence eating behaviors based on the preferences of the microbiota, which can lead to the host consuming more food eventually resulting in obesity. It has generally been observed that with higher gut microbiome diversity, the microbiota will spend energy and resources on competing with other microbiota and less on manipulating the host. The opposite is seen with lower gut microbiome diversity, and these microbiotas may work together for to create host food cravings.[74]
Additionally, the liver plays a dominant role in blood glucose homeostasis by maintaining a balance between the uptake and storage of glucose through the metabolic pathways of glycogenesis and gluconeogenesis. In recent studies, it is illustrated that intestinal lipids regulate glucose homeostasis involving a gut-brain-liver axis. The direct administration of lipids into the upper intestine increases the long chain fatty acyl-coenzyme A (LCFA-CoA) levels in the upper intestines and suppresses glucose production even under sub diaphragmatic vagotomy or gut vagal deafferentation. This interrupts the neural connection between the brain and the gut and blocks the upper intestinal lipids’ ability to inhibit glucose production. The gut-brain-liver axis and gut microbiota composition can regulate the glucose homeostasis in the liver and provide potential therapeutic methods to treat obesity and diabetes.[75]
Just as gut flora can function in a feedback loop that can drive the development of obesity, there is evidence that restricting intake of calories (i.e. dieting) can drive changes to the composition of the gut flora.[70]
Liver disease
As the liver is fed directly by the portal vein, whatever crosses the intestinal epithelium and the intestinal mucosal barrier enters the liver, as do cytokines generated there.[76] Dysbiosis in the gut flora has been linked with the development of cirrhosis and non-alcoholic fatty liver disease.[76]
Systemic infections
Normally-commensal bacteria can be very harmful to the host if they get outside of the intestinal tract.[4][5] Translocation, which occurs when bacteria leave the gut through its mucosal lining, the border between the lumen of the gut and the inside of the body, can occur in a number of different diseases, and can be caused by too much growth of bacteria in the small intestine, reduced immunity of the host, or increased gut lining permeability.[5]
If the gut is perforated, bacteria can invade the body, causing a potentially fatal infection. Aerobic bacteria can make an infection worse by using up all available oxygen and creating an environment favorable to anaerobes.[2]:715
Cancer
Some genera of bacteria, such as Bacteroides and Clostridium, have been associated with an increase in tumor growth rate, while other genera, such as Lactobacillus and Bifidobacteria, are known to prevent tumor formation.[9]
Neuropsychiatric
Interest in the relationship between gut flora and neuropsychiatric issues was sparked by a 2004 study showing that germ-free mice showed an exaggerated HPA axis response to stress compared to non-GF laboratory mice.[42] As of January 2016, most of the work that has been done on the role of gut flora in the gut-brain axis had been conducted in animals, or characterizing the various neuroactive compounds that gut flora can produce, and studies with humans measuring differences between people with various psychiatric and neurological differences, or changes to gut flora in response to stress, or measuring effects of various probiotics (dubbed "psychobiotics in this context), had generally been small and could not be generalized; whether changes to gut flora are a result of disease, a cause of disease, or both in any number of possible feedback loops in the gut-brain axis, remained unclear.[42][52]
A systematic review from 2016 examined the preclinical and small human trials that have been conducted with certain commercially available strains of probiotic bacteria and found that among those tested, Bifidobacterium and Lactobacillus genera (B. longum, B. breve, B. infantis, L. helveticus, L. rhamnosus, L. plantarum, and L. casei), had the most potential to be useful for certain central nervous system disorders.[8]
Other animals
Aside from mammals, some insects also possess complex and diverse gut microbiota that play key nutritional roles.[77] Microbial communities associated termites can constitute a majority of the weight of the individuals and perform important roles in the digestion of lignocellulose and nitrogen fixation.[78] These communities are host-specific, and closely related insect species share comparable similarities in gut microbiota composition.[79][80] In cockroaches, gut microbiota have been shown to assemble in a deterministic fashion, irrespective of the inoculum;[81] the reason for this host-specific assembly remains unclear. Bacterial communities associated with insects like termites and cockroaches are determined by a combination of forces, primarily diet, but there is some indication that host phylogeny may also be playing a role in the selection of lineages.[79][80]
For more than 51 years we have known that the administration of low doses of antibacterial agents promotes the growth of farm animals to increase weight gain.[56]
In a study performed on mice by Ilseung Cho,[56] the ratio of Firmicutes and Lachnospiraceae was significantly elevated in animals treated with subtherapeutic doses of different antibiotics. By analyzing the caloric content of faeces and the concentration of small chain fatty acids (SCFAs) in the GI tract, they concluded that the changes in the composition of microbiota lead to an increased capacity to extract calories from otherwise indigestible constituents, and to an increased production of SCFAs. These findings provide evidence that antibiotics perturb not only the composition of the GI microbiome but also its metabolic capabilities, specifically with respect to SCFAs.[56]
See also
- Colonisation resistance
- List of human flora
- List of microbiota species of the lower reproductive tract of women
- Skin flora
- Verotoxin-producing Escherichia coli
Sources and notes
- 1 2 3 Saxena, R.; Sharma, V.K (2016). "A Metagenomic Insight Into the Human Microbiome: Its Implications in Health and Disease". In D. Kumar; S. Antonarakis. Medical and Health Genomics. Elsevier Science. p. 117. doi:10.1016/B978-0-12-420196-5.00009-5. ISBN 978-0-12-799922-7.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Sherwood, Linda; Willey, Joanne; Woolverton, Christopher (2013). Prescott's Microbiology (9th ed.). New York: McGraw Hill. pp. 713–721. ISBN 9780073402406. OCLC 886600661.
- 1 2 3 4 5 Quigley EM (2013). "Gut bacteria in health and disease". Gastroenterol Hepatol (N Y). 9: 560–9. PMC 3983973
 . PMID 24729765.
. PMID 24729765. - 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Sommer F, Bäckhed F (2013). "The gut microbiota—masters of host development and physiology". Nat Rev Microbiol. 11 (4): 227–38. doi:10.1038/nrmicro2974. PMID 23435359.
- 1 2 3 4 Faderl M; et al. (Apr 2015). "Keeping bugs in check: The mucus layer as a critical component in maintaining intestinal homeostasis". IUBMB Life. 67 (4): 275–85. doi:10.1002/iub.1374. PMID 25914114.
- 1 2 3 4 5 6 Clarke G; et al. (Aug 2014). "Minireview: Gut microbiota: the neglected endocrine organ". Mol Endocrinol. 28 (8): 1221–38. doi:10.1210/me.2014-1108. PMID 24892638.
- 1 2 3 4 5 6 7 Shen S, Wong CH (2016). "Bugging inflammation: role of the gut microbiota". Clin Transl Immunology (Review). 5 (4): e72. doi:10.1038/cti.2016.12. PMC 4855262
 . PMID 27195115.
. PMID 27195115. - 1 2 3 Wang H, Lee IS, Braun C, Enck P (July 2016). "Effect of probiotics on central nervous system functions in animals and humans - a systematic review". J. Neurogastroenterol Motil. doi:10.5056/jnm16018. PMID 27413138.
We reviewed the effect of probiotics on the central nervous system in randomized controlled trials in animals and humans, and analyzed the possibility of translating animal models to human studies because few human studies have been conducted to date. According to the qualitative analyses of current studies, we can provisionally draw the conclusion that B. longum, B. breve, B. infantis, L. helveticus, L. rhamnosus, L. plantarum, and L. casei were most effective in improving CNS function, including psychiatric disease-associated functions (anxiety, depression, mood, stress response) and memory abilities.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Guarner, F; Malagelada, J (2003). "Gut flora in health and disease". The Lancet. 361 (9356): 512–9. doi:10.1016/S0140-6736(03)12489-0. PMID 12583961.
- 1 2 3 4 5 6 7 Sears, Cynthia L. (2005). "A dynamic partnership: Celebrating our gut flora". Anaerobe. 11 (5): 247–51. doi:10.1016/j.anaerobe.2005.05.001. PMID 16701579.
- 1 2 3 4 5 6 7 8 9 10 11 Beaugerie, Laurent; Petit, Jean-Claude (2004). "Antibiotic-associated diarrhoea". Best Practice & Research Clinical Gastroenterology. 18 (2): 337–52. doi:10.1016/j.bpg.2003.10.002. PMID 15123074.
- ↑ Stephen, A. M.; Cummings, J. H. (1980). "The Microbial Contribution to Human Faecal Mass". Journal of Medical Microbiology. 13 (1): 45–56. doi:10.1099/00222615-13-1-45. PMID 7359576.
- ↑ Lozupone, Catherine A.; Stombaugh, Jesse I.; Gordon, Jeffrey I.; Jansson, Janet K.; Knight, Rob (2012). "Diversity, stability and resilience of the human gut microbiota". Nature. 489 (7415): 220–30. Bibcode:2012Natur.489..220L. doi:10.1038/nature11550. PMC 3577372
 . PMID 22972295.
. PMID 22972295. - ↑ Qin, Junjie; Li, Ruiqiang; Raes, Jeroen; Arumugam, Manimozhiyan; Burgdorf, Kristoffer Solvsten; Manichanh, Chaysavanh; Nielsen, Trine; Pons, Nicolas; Levenez, Florence; Yamada, Takuji; Mende, Daniel R.; Li, Junhua; Xu, Junming; Li, Shaochuan; Li, Dongfang; Cao, Jianjun; Wang, Bo; Liang, Huiqing; Zheng, Huisong; Xie, Yinlong; Tap, Julien; Lepage, Patricia; Bertalan, Marcelo; Batto, Jean-Michel; Hansen, Torben; Le Paslier, Denis; Linneberg, Allan; Nielsen, H. Bjørn; Pelletier, Eric; Renault, Pierre (2010). "A human gut microbial gene catalogue established by metagenomic sequencing". Nature. 464 (7285): 59–65. Bibcode:2010Natur.464...59.. doi:10.1038/nature08821. PMC 3779803
 . PMID 20203603.
. PMID 20203603. - ↑ Shanahan, Fergus (2002). "The host–microbe interface within the gut". Best Practice & Research Clinical Gastroenterology. 16 (6): 915–31. doi:10.1053/bega.2002.0342. PMID 12473298.
- ↑ Tap, Julien; Mondot, Stanislas; Levenez, Florence; Pelletier, Eric; Caron, Christophe; Furet, Jean-Pierre; Ugarte, Edgardo; Muñoz-Tamayo, Rafael; Paslier, Denis L. E.; Nalin, Renaud; Dore, Joel; Leclerc, Marion (2009). "Towards the human intestinal microbiota phylogenetic core". Environmental Microbiology. 11 (10): 2574–84. doi:10.1111/j.1462-2920.2009.01982.x. PMID 19601958.
- 1 2 O'Hara, Ann M; Shanahan, Fergus (2006). "The gut flora as a forgotten organ". EMBO Reports. 7 (7): 688–93. doi:10.1038/sj.embor.7400731. PMC 1500832
 . PMID 16819463.
. PMID 16819463. - ↑ Khanna S, Tosh PK (January 2014). "A clinician's primer on the role of the microbiome in human health and disease". Mayo Clin. Proc. 89 (1): 107–14. doi:10.1016/j.mayocp.2013.10.011. PMID 24388028.
- 1 2 Cui L, Morris A, Ghedin E (July 2013). "The human mycobiome in health and disease". Genome Med. 5 (7): 63. doi:10.1186/gm467. PMC 3978422
 . PMID 23899327.
. PMID 23899327. Figure 2: Distribution of fungal genera in different body sites
- ↑ Erdogan A, Rao SS (April 2015). "Small intestinal fungal overgrowth". Curr Gastroenterol Rep. 17 (4): 16. doi:10.1007/s11894-015-0436-2. PMID 25786900.
- 1 2 Arumugam, Manimozhiyan; Raes, Jeroen; Pelletier, Eric; Le Paslier, Denis; Yamada, Takuji; Mende, Daniel R.; Fernandes, Gabriel R.; Tap, Julien; Bruls, Thomas; Batto, Jean-Michel; Bertalan, Marcelo; Borruel, Natalia; Casellas, Francesc; Fernandez, Leyden; Gautier, Laurent; Hansen, Torben; Hattori, Masahira; Hayashi, Tetsuya; Kleerebezem, Michiel; Kurokawa, Ken; Leclerc, Marion; Levenez, Florence; Manichanh, Chaysavanh; Nielsen, H. Bjørn; Nielsen, Trine; Pons, Nicolas; Poulain, Julie; Qin, Junjie; Sicheritz-Ponten, Thomas; Tims, Sebastian (2011). "Enterotypes of the human gut microbiome". Nature. 473 (7346): 174–80. Bibcode:2011Natur.473..174.. doi:10.1038/nature09944. PMC 3728647
 . PMID 21508958.
. PMID 21508958. - 1 2 Wu, G. D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S. A.; Bewtra, M.; Knights, D.; Walters, W. A.; Knight, R.; Sinha, R.; Gilroy, E.; Gupta, K.; Baldassano, R.; Nessel, L.; Li, H.; Bushman, F. D.; Lewis, J. D. (2011). "Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes". Science. 334 (6052): 105–8. Bibcode:2011Sci...334..105W. doi:10.1126/science.1208344. PMC 3368382
 . PMID 21885731.
. PMID 21885731. - ↑ Zimmer, Carl (April 20, 2011). "Bacteria Divide People Into 3 Types, Scientists Say". The New York Times. Retrieved April 21, 2011.
a group of scientists now report just three distinct ecosystems in the guts of people they have studied.
- ↑ Kenneth Todar (2012). "The Normal Bacterial Flora of Humans". Todar's Online Textbook of Bacteriology. Retrieved June 25, 2016.
- ↑ Quigley, Eamonn M M; Rodrigo Quera (February 2006). "Small intestinal bacterial overgrowth: roles of antibiotics, prebiotics, and probiotics". Gastroenterology. 130 (2 Suppl 1): S78–90. doi:10.1053/j.gastro.2005.11.046. ISSN 0016-5085. PMID 16473077.
- 1 2 University of Glasgow. 2005. The normal gut flora. Available through web archive. Accessed May 22, 2008
- 1 2 Steinhoff, U (2005). "Who controls the crowd? New findings and old questions about the intestinal microflora". Immunology Letters. 99 (1): 12–6. doi:10.1016/j.imlet.2004.12.013. PMID 15894105.
- 1 2 3 4 5 6 7 Gibson, Glenn R. (2004). "Fibre and effects on probiotics (the prebiotic concept)". Clinical Nutrition Supplements. 1 (2): 25–31. doi:10.1016/j.clnu.2004.09.005.
- ↑ Miquel, S; Martín, R; Rossi, O; Bermúdez-Humarán, LG; Chatel, JM; Sokol, H; Thomas, M; Wells, JM; Langella, P (2013). "Faecalibacterium prausnitzii and human intestinal health". Current Opinion in Microbiology. 16 (3): 255–61. doi:10.1016/j.mib.2013.06.003. PMID 23831042.
- ↑ Scarpellini E, Ianiro G, Attili F, Bassanelli C, De Santis A, Gasbarrini A (2015). "The human gut microbiota and virome: Potential therapeutic implications". Dig Liver Dis (Review). 47 (12): 1007–12. doi:10.1016/j.dld.2015.07.008. PMID 26257129.
- ↑ Ley, Ruth E. "Obesity and the Human Microbiome." Current Opinion in Gastroenterology 26.1 (2010): 5-11. Wolters Kluwer Health. doi: 10.1097/MOG.0b013e328333d751
- 1 2 Gerritsen, Jacoline; Smidt, Hauke; Rijkers, Ger; de Vos, Willem (27 May 2011). "Intestinal microbiota in human health and disease: the impact of probiotics". Genes & Nutritions. 6 (3): 209–240. doi:10.1007/s12263-011-0229-7. PMC 3145058
 . PMID 21617937.
. PMID 21617937. - 1 2 3 4 5 6 Yatsunenko, T.; Rey, F. E.; Manary, M. J.; Trehan, I.; Dominguez-Bello, M. G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R. N.; Anokhin, A. P.; Heath, A. C.; Warner, B.; Reeder, J.; Kuczynski, J.; Caporaso, J. G.; Lozupone, C. A.; Lauber, C.; Clemente, J. C.; Knights, D.; Knight, R.; Gordon, J. I. (2012). "Human gut microbiome viewed across age and geography". Nature. 486 (7402): 222–227. Bibcode:2012Natur.486..222Y. doi:10.1038/nature11053. PMC 3376388
 . PMID 22699611.
. PMID 22699611. - ↑ Jonkers, Daisy M.A.E. (2016). "Microbial perturbations and modulation in conditions associated with malnutrition and malabsorption". Best Practice & Research Clinical Gastroenterology. 30 (2): 161–172. doi:10.1016/j.bpg.2016.02.006. PMID 27086883.
- ↑ Million, Matthieu; Diallo, Aldiouma; Raoult, Didier (2016). "Gut microbiota and malnutrition" (PDF). Microbial Pathogenesis. doi:10.1016/j.micpath.2016.02.003. PMID 26853753.
- ↑ Rytter, Maren Johanne Heilskov; Kolte, Lilian; et al. (2014). "The Immune System in Children with Malnutrition". PLoS. 9 (8): e105017. Bibcode:2014PLoSO...9j5017R. doi:10.1371/journal.pone.0105017. PMC 4143239
 . PMID 25153531.
. PMID 25153531. - ↑ De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J. B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. (2010). "Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa". Proc. Natl. Acad. Sci. U.S.A. 107 (33): 14691–14696. Bibcode:2010PNAS..10714691D. doi:10.1073/pnas.1005963107. PMC 2930426
 . PMID 20679230.
. PMID 20679230. - 1 2 Matamoros S; et al. (2013). "Development of intestinal microbiota in infants and its impact on health.". Trends Microbiol. 21 (4): 167–73. doi:10.1016/j.tim.2012.12.001. PMID 23332725.
- ↑ Coppa, Giovanni V; Bruni, Stefano; Morelli, Lorenzo; Soldi, Sara; Gabrielli, Orazio (2004). "The First Prebiotics in Humans". Journal of Clinical Gastroenterology. 38 (6 Suppl): S80–3. doi:10.1097/01.mcg.0000128926.14285.25. PMID 15220665.
- ↑ Coppa, G.V.; Zampini, L.; Galeazzi, T.; Gabrielli, O. (2006). "Prebiotics in human milk: A review". Digestive and Liver Disease. 38: S291–4. doi:10.1016/S1590-8658(07)60013-9. PMID 17259094.
- ↑ Fanaro, S; Chierici, R; Guerrini, P; Vigi, V (2003). "Intestinal microflora in early infancy: Composition and development". Acta paediatrica. 91 (441): 48–55. PMID 14599042.
- 1 2 3 4 5 6 7 8 Wang Y, Kasper LH (2014). "The role of microbiome in central nervous system disorders". Brain Behav Immun. 38: 1–12. doi:10.1016/j.bbi.2013.12.015. PMID 24370461.
- 1 2 Yoon MY, Lee K, Yoon SS (2014). "Protective role of gut commensal microbes against intestinal infections". J Microbiol. 52 (12): 983–9. doi:10.1007/s12275-014-4655-2. PMID 25467115.
- ↑ Reinoso Webb C (2016). "Protective and pro-inflammatory roles of intestinal bacteria". Pathophysiology (Review). 23 (2): 67–80. doi:10.1016/j.pathophys.2016.02.002. PMID 26947707.
- ↑ Mantis NJ, Rol N, Corthésy B (2011). "Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut". Mucosal Immunol. 4 (6): 603–11. doi:10.1038/mi.2011.41. PMC 3774538
 . PMID 21975936.
. PMID 21975936. - 1 2 3 4 5 6 7 8 9 Zhang LS, Davies SS (April 2016). "Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions". Genome Med. 8 (1): 46. doi:10.1186/s13073-016-0296-x. PMC 4840492
 . PMID 27102537.
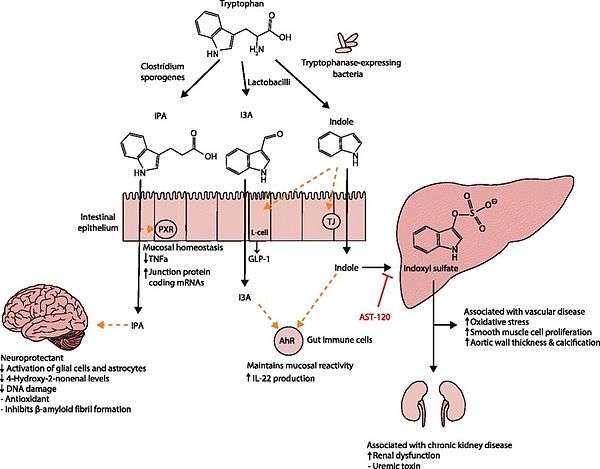
. PMID 27102537. Lactobacillus spp. convert tryptophan to indole-3-aldehyde (I3A) through unidentified enzymes [125]. Clostridium sporogenes convert tryptophan to IPA [6], likely via a tryptophan deaminase. ... IPA also potently scavenges hydroxyl radicals
Table 2: Microbial metabolites: their synthesis, mechanisms of action, and effects on health and disease
Figure 1: Molecular mechanisms of action of indole and its metabolites on host physiology and disease - ↑ Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G (2009). "Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites". Proc. Natl. Acad. Sci. U.S.A. 106 (10): 3698–703. doi:10.1073/pnas.0812874106. PMC 2656143
 . PMID 19234110.
. PMID 19234110. Production of IPA was shown to be completely dependent on the presence of gut microflora and could be established by colonization with the bacterium Clostridium sporogenes.
IPA metabolism diagram - ↑ "3-Indolepropionic acid". Human Metabolome Database. University of Alberta. Retrieved 12 October 2015.
Indole-3-propionate (IPA), a deamination product of tryptophan formed by symbiotic bacteria in the gastrointestinal tract of mammals and birds. 3-Indolepropionic acid has been shown to prevent oxidative stress and death of primary neurons and neuroblastoma cells exposed to the amyloid beta-protein in the form of amyloid fibrils, one of the most prominent neuropathologic features of Alzheimer's disease. 3-Indolepropionic acid also shows a strong level of neuroprotection in two other paradigms of oxidative stress. (PMID 10419516 )
Origin: • Endogenous • Microbial - ↑ Chyan YJ, Poeggeler B, Omar RA, Chain DG, Frangione B, Ghiso J, Pappolla MA (1999). "Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid". J. Biol. Chem. 274 (31): 21937–42. doi:10.1074/jbc.274.31.21937. PMID 10419516.
[Indole-3-propionic acid (IPA)] has previously been identified in the plasma and cerebrospinal fluid of humans, but its functions are not known. ... In kinetic competition experiments using free radical-trapping agents, the capacity of IPA to scavenge hydroxyl radicals exceeded that of melatonin, an indoleamine considered to be the most potent naturally occurring scavenger of free radicals. In contrast with other antioxidants, IPA was not converted to reactive intermediates with pro-oxidant activity.
- ↑ Mayer EA, Knight R, Mazmanian SK; et al. (2014). "Gut microbes and the brain: paradigm shift in neuroscience" (PDF). J Neurosci. 34: 15490–15496. doi:10.1523/JNEUROSCI.3299-14.2014. PMC 4228144
 . PMID 25392516.
. PMID 25392516. - 1 2 3 Dinan, T.G; Cryan, 2015 (2015). "The impact of gut microbiota on brain and behavior: implications for psychiatry". Curr Opin Clin Nutr Metab Care. 18: 552–558. doi:10.1097/MCO.0000000000000221. PMID 26372511.
- 1 2 3 4 5 Schneiderhan J, Master-Hunter T, Locke A (2016). "Targeting gut flora to treat and prevent disease". J Fam Pract. 65: 34–8. PMID 26845162.
- 1 2 3 Carman, Robert J.; Simon, Mary Alice; Fernández, Haydée; Miller, Margaret A.; Bartholomew, Mary J. (2004). "Ciprofloxacin at low levels disrupts colonization resistance of human fecal microflora growing in chemostats". Regulatory Toxicology and Pharmacology. 40 (3): 319–26. doi:10.1016/j.yrtph.2004.08.005. PMID 15546686.
- ↑ Brandt, Lawrence J.; Borody, Thomas Julius; Campbell, Jordana (2011). "Endoscopic Fecal Microbiota Transplantation". Journal of Clinical Gastroenterology. 45 (8): 655–7. doi:10.1097/MCG.0b013e3182257d4f. PMID 21716124.
- ↑ Knight, DJW; Girling, KJ (2003). "Gut flora in health and disease". The Lancet. 361 (9371): 512–9. doi:10.1016/S0140-6736(03)13438-1. PMID 12781578.
- 1 2 3 4 Cho, I.; Yamanishi, S.; Cox, L.; Methé, B. A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; Li, H.; Alekseyenko, A. V.; Blaser, M. J. (2012). "Antibiotics in early life alter the murine colonic microbiome and adiposity". Nature. 488 (7413): 621–6. Bibcode:2012Natur.488..621C. doi:10.1038/nature11400. PMC 3553221
 . PMID 22914093.
. PMID 22914093. - ↑ Baker, Monya (2012). "Pregnancy alters resident gut microbes". Nature. doi:10.1038/nature.2012.11118.
- ↑ Hill, C; Guarner, F; Reid, G; Gibson, GR; Merenstein, DJ; Pot, B; Morelli, L; Canani, RB; Flint, HJ; Salminen, S; Calder, PC; Sanders, ME (August 2014). "Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic". Nature reviews. Gastroenterology & hepatology. 11 (8): 506–14. doi:10.1038/nrgastro.2014.66. PMID 24912386.
- ↑ Rijkers GT, de Vos WM, Brummer RJ, Morelli L, Corthier G, Marteau P; De Vos; Brummer; Morelli; Corthier; Marteau (2011). "Health benefits and health claims of probiotics: Bridging science and marketing". British Journal of Nutrition. 106 (9): 1291–6. doi:10.1017/S000711451100287X. PMID 21861940.
- ↑ Hutkins RW, Krumbeck JA, Bindels LB, Cani PD, Fahey G Jr., Goh YJ, Hamaker B, Martens EC, Mills DA, Rastal RA, Vaughan E, Sanders ME (2016). "Prebiotics: why definitions matter". Curr Opin Biotechnol. 37: 1–7. doi:10.1016/j.copbio.2015.09.001. PMC 4744122
 . PMID 26431716.
. PMID 26431716. - ↑ Pandey KR, Naik SR, Vakil BV (2015). "Probiotics, prebiotics and synbiotics- a review". J Food Sci Technol. (Review). 52 (12): 7577–87. doi:10.1007/s13197-015-1921-1. PMID 26604335.
- ↑ Broeckx G; et al. (2016). "Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics". Int J Pharm. (Review). 505 (1–2): 303–18. doi:10.1016/j.ijpharm.2016.04.002. PMID 27050865.
- ↑ Sleator RD, Hill C (2009). "Rational design of improved pharmabiotics". J Biomed Biotechnol. 2009: 275–287. doi:10.1155/2009/275287. PMC 2742647
 . PMID 19753318.
. PMID 19753318. - ↑ Patterson E; et al. (2014). "Gut microbiota, the pharmabiotics they produce and host health". Proc Nutr Soc. 73 (4): 477–89. doi:10.1017/S0029665114001426. PMID 25196939.
- ↑ Ford, Alexander C; Quigley, Eamonn M M; Lacy, Brian E; Lembo, Anthony J; Saito, Yuri A; Schiller, Lawrence R; Soffer, Edy E; Spiegel, Brennan M R; Moayyedi, Paul (2014). "Efficacy of Prebiotics, Probiotics, and Synbiotics in Irritable Bowel Syndrome and Chronic Idiopathic Constipation: Systematic Review and Meta-analysis". The American Journal of Gastroenterology. 109 (10): 1547–1561. doi:10.1038/ajg.2014.202. ISSN 0002-9270. PMID 25070051.
- 1 2 Ghouri, Yezaz A; Richards, David M; Rahimi, Erik F; Krill, Joseph T; Jelinek, Katherine A; DuPont, Andrew W (2014). "Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease". Clin Exp Gastroenterol. 7: 473–487. doi:10.2147/CEG.S27530. PMC 4266241
 . PMID 25525379.
. PMID 25525379. - ↑ Yu CG, Huang Q. (2013). "Recent progress on the role of gut microbiota in the pathogenesis of inflammatory bowel disease". J Dig Dis. 14 (10): 513–7. doi:10.1111/1751-2980.12087. PMID 23848393.
- ↑ Burisch, Johan; Jess, Tine; Martinato, Matteo; Lakatos, Peter L. (2013). "The burden of inflammatory bowel disease in Europe". Journal of Crohn's and Colitis. 7 (4): 322–337. doi:10.1016/j.crohns.2013.01.010. ISSN 1873-9946. PMID 23395397.
- ↑ Blandino G; et al. (2016). "Impact of gut microbiota on diabetes mellitus". Diabetes Metab. (Review). doi:10.1016/j.diabet.2016.04.004. PMID 27179626. pii: S1262-3636(16)30396-2
- 1 2 3 Boulangé CL; et al. (2016). "Impact of the gut microbiota on inflammation, obesity, and metabolic disease". Genome Med. (Review). 8 (1): 42. doi:10.1186/s13073-016-0303-2. PMC 4839080
 . PMID 27098727.
. PMID 27098727. - ↑ Ipci K; et al. (2016). "The possible mechanisms of the human microbiome in allergic diseases". Eur Arch Otorhinolaryngol. (Review). doi:10.1007/s00405-016-4058-6. PMID 27115907.
- ↑ Spiller R (2016). "Irritable bowel syndrome: new insights into symptom mechanisms and advances in treatment". F1000Research. 5 (5(F1000 Faculty Rev)): 780. doi:10.12688/f1000research.7992.1. PMC 4856111
 . PMID 27158477.
. PMID 27158477. - ↑ Mazidi M, et al. Gut microbiome and metabolic syndrome. Diabetes Metab Syndr. 2016 Feb 11. Review. doi:10.1016/j.dsx.2016.01.024 PMID 26916014
- ↑ Alcock, J.; Maley, C.C.; Aktipis, C.A. (2014). "Is eating behavior manipulated by gastrointestinal microbiota? Evolutionary pressures and potential mechanisms". BioEssays. 36: 940–949. doi:10.1002/bies.201400071. PMC 4270213
 . PMID 25103109.
. PMID 25103109. - ↑ Chen, X; D'Souza, R; Hong, ST (2013). "The role of gut microbiota in the gut-brain axis: current challenges and perspectives". Protein & Cell. 4 (6): 403–14. doi:10.1007/s13238-013-3017-x. PMID 23686721.
- 1 2 Minemura M, Shimizu Y (2015). "Gut microbiota and liver diseases". World J Gastroenterol. 21 (6): 1691–702. doi:10.3748/wjg.v21.i6.1691. PMC 4323444
 . PMID 25684933.
. PMID 25684933. - ↑ Engel, P.; Moran, N. (2013). "The gut microbiota of insects–diversity in structure and function". FEMS Microbiology Reviews. 5 (5): 699–735. doi:10.1111/1574-6976.12025.
- ↑ Brune, A. (2014). "Symbiotic digestion of lignocellulose in termite guts". Nature Review Microbiology. 12 (3): 168–180. doi:10.1038/nrmicro3182.
- 1 2 Dietrich, C.; Köhler, T.; Brune, A. (2014). "The cockroach origin of the termite gut microbiota: patterns in bacterial community structure reflect major evolutionary events". Applied and Environmental Microbiology. 80 (7): 2261–2269. doi:10.1128/AEM.04206-13. PMC 3993134
 . PMID 24487532.
. PMID 24487532. - 1 2 Mikaelyan, A.; Dietrich, C.; Köhler, T.; Poulsen, M.; Sillam-Dussès, D.; Brune, A. (2015). "Diet is the primary determinant of bacterial community structure in the guts of higher termites". Molecular Ecology. 24 (20): 5824–5895. doi:10.1111/mec.13376. PMID 26348261.
- ↑ Mikaelyan, A.; Thompson, C.; Hofer, M.; Brune, A. (2016). "The deterministic assembly of complex bacterial communities in germ-free cockroach guts". Applied and Environmental Microbiology. 82 (4): 1256–1263. doi:10.1128/AEM.03700-15. PMID 26655763.
Further reading
- Review articles
- Maranduba, CM; De Castro, SB; de Souza, GT; Rossato, C; da Guia, FC; Valente, MA; Rettore, JV; Maranduba, CP; de Souza, CM; do Carmo, AM; Macedo, GC; Silva, FS (2015). "Intestinal Microbiota as Modulators of the Immune System and Neuroimmune System: Impact on the Host Health and Homeostasis". Journal of Immunology Research. 2015: 931574. doi:10.1155/2015/931574. PMC 4352473
 . PMID 25759850.
. PMID 25759850. - De Preter, Vicky; Hamer, Henrike M; Windey, Karen; Verbeke, Kristin (2011). "The impact of pre- and/or probiotics on human colonic metabolism: Does it affect human health?". Molecular Nutrition & Food Research. 55 (1): 46–57. doi:10.1002/mnfr.201000451. PMID 21207512.
- Prakash, Satya; Rodes, Laetitia; Coussa-Charley, Michael; Tomaro-Duchesneau, Catherine; Tomaro-Duchesneau, Catherine; Coussa-Charley; Rodes (2011). "Gut microbiota: Next frontier in understanding human health and development of biotherapeutics". Biologics: Targets and Therapy. 5: 71–86. doi:10.2147/BTT.S19099. PMC 3156250
 . PMID 21847343.
. PMID 21847343. - Wu, G. D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S. A.; Bewtra, M.; Knights, D.; Walters, W. A.; Knight, R.; Sinha, R.; Gilroy, E.; Gupta, K.; Baldassano, R.; Nessel, L.; Li, H.; Bushman, F. D.; Lewis, J. D. (2011). "Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes". Science. 334 (6052): 105–8. Bibcode:2011Sci...334..105W. doi:10.1126/science.1208344. PMC 3368382
 . PMID 21885731.
. PMID 21885731.
External links
| Wikispecies has information related to: Microbiota |