Ovarian follicle
| Ovarian follicle | |
|---|---|
.jpg) Histology section of a mature ovarian follicle. The oocyte is the large, round, pink-staining cell at top center of the image. | |
| Details | |
| Precursor | Cortical cords |
| Identifiers | |
| Latin | Folliculus ovaricus |
| MeSH | A05.360.319.114.630.535 |
| TA | A09.1.01.013 |
| FMA | 18641 |
An ovarian follicle is a roughly spheroid cellular aggregation set found in the ovaries. It secretes hormones that influence stages of the menstrual cycle. Women begin puberty with about 400,000 follicles,[1] each with the potential to release an egg cell (ovum) at ovulation for fertilization.[2] These eggs are developed only once every menstrual cycle.
Structure

Ovarian follicles are the basic units of female reproductive biology. Each of them contains a single oocyte (immature ovum or egg cell). These structures are periodically initiated to grow and develop, culminating in ovulation of usually a single competent oocyte in humans.[3] They also consist of granulosa cells and theca of follicle.
Oocyte
Once a month, one of the ovaries releases a mature egg (ovum), known as an oocyte. A follicle is an anatomical structure in which the primary oocyte develops. The nucleus of such an oocyte is called a germinal vesicle[4] (see picture).
Cumulus oophorus
Cumulus oophorus is a cluster of cells (called cumulus cells) that surround the oocyte both in the ovarian follicle and after ovulation.
Membrana granulosa
It contains numerous granulosa cells.
Granulosa cell
Granulosa cells or follicular cells are cells that surround the oocyte within the follicle; their numbers increase directly in response to heightened levels of circulating gonadotropins or decrease in response to testosterone. They also produce peptides involved in ovarian hormone synthesis regulation. Follicle-stimulating hormone (FSH) induces granulosa cells to express luteinizing hormone (LH) receptors on their surfaces; when circulating LH binds to these receptors, proliferation stops.[5]
Theca of follicle
The granulosa cells, in turn, are enclosed in a thin layer of extracellular matrix – the follicular basement membrane or basal lamina (fibro-vascular coat in picture). Outside the basal lamina, the layers theca interna and theca externa are found.
Development
Primordial follicles are indiscernible to the naked eye. However, these eventually develop into primary, secondary and tertiary vesicular follicles. Tertiary vesicular follicles (also called "mature vesicular follicles" or "ripe vesicular follicles") are sometimes called Graafian follicles (after Regnier de Graaf).
In humans, oocytes are established in the ovary before birth and may lie dormant awaiting initiation for up to 50 years.[6]
After rupturing, the follicle is turned into a corpus luteum.
Development of oocytes in ovarian follicles
In a larger perspective, the whole folliculogenesis from primordial to preovulatory follicle is located in the stage of meiosis I of ootidogenesis in oogenesis.
Embryonic development in males and females follows a common pathway before gametogenesis. Once gametogonia enter the gonadal ridge, however, they attempt to associate with these somatic cells. Development proceeds and the gametogonia turn into oogonia, which become fully surrounded by a layer of cells (pre-granulosa cells).
The Oogonia multiply by dividing mitotically; this proliferation ends when the oogonia enter meiosis. The amount of time that oogonia multiply by mitosis is not species specific. In the human fetus, cells undergoing mitosis are seen until the second and third trimester of pregnancy.[7][8] After beginning the meiotic process, the oogonia (now called primary oocytes) can no longer replicate. Therefore, the total number of gametes is established at this time. Once the primary oocytes stop dividing the cells enter a prolonged ‘resting phase’. This ‘resting phase’ or dictyate stage can last anywhere up to fifty years in the human.
For several primary oocytes that complete meiosis I each month, only one or a few functional oocyte, the dominant follicles, completes maturation and undergoes ovulation. The other follicles that begin to mature will regress and become atretic follicles, eventually deteriorating.
The primary oocyte turns into a secondary oocyte in mature ovarian follicles. Unlike the sperm, the egg is arrested in the secondary stage of meiosis until fertilization.
Upon fertilization by sperm, the secondary oocyte continues the second part of meiosis and becomes a zygote.
Clinical significance
Any ovarian follicle that is larger than about two centimeters is termed an ovarian cyst.
Ovarian function may be measured by gynecologic ultrasonography of follicular volume. Presently, ovarian follicle volumes can be measured rapidly and automatically from three-dimensionally reconstructed ultrasound images.[9]
Rupture of the follicle can result in abdominal pain (mittelschmerz) and is to be considered in the differential diagnosis in women of childbearing age.[10]
Cryopreservation and culture tissue after cryopreservation. Cryopreservation of ovarian tissue is of interest to women who want to preserve their reproductive function beyond the natural limit, or whose reproductive potential is threatened by cancer therapy,[11] for example in hematologic malignancies or breast cancer.[12]
For in vitro culture of follicles, there are various techniques to optimize the growth of follicles, including the use of defined media, growth factors and three-dimensional extracellular matrix support.[13] Molecular methods and immunoassay can evaluate stage of maturation and guide adequate differentiation.[13] Animal studies have generally showed correct imprinted DNA methylation establishment in oocytes resulting from follicle culture.[14]
Additional images
- Pre-antral follicle
- Graafian follicles
.jpg) Primordial ovarian follicle. The oocyte is surrounded by a single layer of flat granulosa cells.
Primordial ovarian follicle. The oocyte is surrounded by a single layer of flat granulosa cells.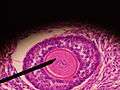 A histological slide of a human primary ovarian follicle in greater magnification.
A histological slide of a human primary ovarian follicle in greater magnification.
References
- ↑ David Krogh (2010), Biology: A Guide to the Natural World, Benjamin-Cummings Publishing Company, p. 638, ISBN 978-0-321-61655-5
- ↑ "What Is an Ovarian Follicle?". wiseGEEK.org. wiseGEEK. Retrieved 24 May 2015.
- ↑ Luijkx, Tim. "Ovarian follicle". radiopaedia.org. radiopaedia.org. Retrieved 24 May 2015.
- ↑ Biology-online
- ↑ Katz: Comprehensive Gynecology, 5th ed.
- ↑ McGee E. A.; Hsueh A. J. (2000). "Initial and cyclic recruitment of ovarian follicles". Endocrine Reviews. 21 (2): 200–14. doi:10.1210/er.21.2.200. PMID 10782364.
- ↑ Baker, T. G. (1982). Oogenesis and ovulation. In "Book 1: Germ cells and fertilization" (C. R. Austin and R. V. Short, Eds.), pp. 17-45. Cambridge University Press, Cambridge.
- ↑ Byskov, A. G., and Hoyer, P. E. (1988). Embryology of mammalian gonads and ducts. In "The physiology of reproduction" (E. Knobil and J. Neill, Eds.), pp. 265-302. Raven Press, Ltd, New York.
- ↑ Salama S, Arbo E, Lamazou F, Levailllant JM, Frydman R, Fanchin R (April 2010). "Reproducibility and reliability of automated volumetric measurement of single preovulatory follicles using SonoAVC". Fertil. Steril. 93 (6): 2069–73. doi:10.1016/j.fertnstert.2008.12.115. PMID 19342038.
- ↑ http://www.merck.com/mmpe/sec02/ch011/ch011b.html
- ↑ Isachenko V, Lapidus I, Isachenko E, et al. (2009). "Human ovarian tissue vitrification versus conventional freezing: morphological, endocrinological, and molecular biological evaluation.". Reproduction. 138 (2): 319–27. doi:10.1530/REP-09-0039. PMID 19439559.
- ↑ Oktay K, Oktem O (November 2008). "Ovarian cryopreservation and transplantation for fertility preservation for medical indications: report of an ongoing experience". Fertil. Steril. 93 (3): 762–8. doi:10.1016/j.fertnstert.2008.10.006. PMID 19013568.
- 1 2 Smitz J, Dolmans MM, Donnez J, et al. (February 2010). "Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation". Hum Reprod Update. 16 (4): 395–414. doi:10.1093/humupd/dmp056. PMC 2880913
 . PMID 20124287.
. PMID 20124287. - ↑ Anckaert, E.; De Rycke, M.; Smitz, J. (2012). "Culture of oocytes and risk of imprinting defects". Human Reproduction Update. 19 (1): 52–66. doi:10.1093/humupd/dms042. PMID 23054129.
External links
- Anatomy photo:43:05-0105 at the SUNY Downstate Medical Center - "The Female Pelvis: The Ovary"
- Histology image: 14803loa – Histology Learning System at Boston University
- Slide at fda.gov
- Images at okstate.edu
- Life cycle at gfmer.ch