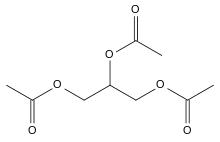
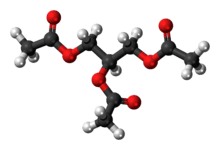
Triacetin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,3-Diacetyloxypropan-2-yl acetate | |
| Other names
Glycerol triacetate[2] | |
| Identifiers | |
| 102-76-1 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:9661 |
| ChEMBL | ChEMBL1489254 |
| ChemSpider | 13835706 |
| ECHA InfoCard | 100.002.775 |
| E number | E1518 (additional chemicals) |
| KEGG | D00384 |
| RTECS number | AK3675000 |
| UNII | XHX3C3X673 |
| |
| |
| Properties | |
| C9H14O6 | |
| Molar mass | 218.21 g·mol−1 |
| Appearance | Oily liquid |
| Density | 1.155 g/cm3[3] |
| Melting point | −78 °C (−108 °F; 195 K) at 760 mmHg[2] |
| Boiling point | 259 °C (498 °F; 532 K) at 760 mmHg[2] |
| 6.1 g/100 mL[2] | |
| Solubility | Miscible in EtOH Soluble in C6H6, (C2H5)2O, acetone[2] |
| Vapor pressure | 0.051 Pa (11.09 °C) 0.267 Pa (25.12 °C) 2.08 Pa (45.05 °C)[4] ln(P/Pa)=22.819-4493/T(K)-807000/T(K)² |
| Refractive index (nD) |
1.4301 (20 °C)[2] 1.4294 (24.5 °C)[4] |
| Viscosity | 23 cP (20 °C)[3] |
| Thermochemistry | |
| 389 J/mol·K[5] | |
| Std molar entropy (S |
458.3 J/mol·K[5] |
| Std enthalpy of formation (ΔfH |
−1330.8 kJ/mol[5] |
| Std enthalpy of combustion (ΔcH |
4211.6 kJ/mol[5] |
| Hazards | |
| S-phrases | S24/25 |
| NFPA 704 | |
| Flash point | 138 °C (280 °F; 411 K)[3] |
| 430 °C (806 °F; 703 K)[3] | |
| Explosive limits | 7.73%[3] |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
1100 mg/kg (mice, oral)[3] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
The triglyceride 1,2,3-triacetoxypropane is more generally known as triacetin and glycerin triacetate. It is the triester of glycerol and acetylating agents, such as acetic acid and acetic anhydride.[6] It is a colorless, viscous and odorless liquid.
Uses
It is an artificial chemical compound,[7] commonly used as a food additive, for instance as a solvent in flavourings, and for its humectant function, with E number E1518 and Australian approval code A1518. It is used as an excipient in pharmaceutical products, where it is used as a humectant, a plasticizer, and as a solvent.[8]
Triacetin can also be used as a fuel additive as an antiknock agent which can reduce engine knocking in gasoline, and to improve cold and viscosity properties of biodiesel.[9]
In a 1994 report released by five top cigarette companies, triacetin was listed as one of the 599 cigarette additives. The triacetin is applied to the filter as a plasticizer.[10]
It has been considered as a possible source of food energy in artificial food regeneration systems on long space missions. It is believed to be safe to get over half of one's dietary energy from triacetin.[11]
Safety
The US Food and Drug Administration has approved it as Generally Recognized as Safe (GRAS) food additive and included it in the database according to the opinion from the Select Committee On GRAS Substances (SCOGS).
- "Three types of acetostearins have been found to be without toxic effects in long-term feeding tests in rats at levels up to 5 g per kg per day. This contrasts with an estimated human consumption of a fraction of a milligram per kg per day. It is recognized that at an even higher feeding level (10 g per kg per day) male rats developed testicular atrophy and female rats, uterine discoloration. However, such a level which would amount to 50 g or more for an infant and 600 g for an adult per day, is vastly higher than would be possible in the consumption of foods to which acetostearins are added for functional purposes."
Triacetin is included in the SCOGS database since 1975.[12]
Triacetin was not toxic to animals in studies of exposure through repeated inhalation over a relatively short period.[13]
References
- ↑ Merck Index, 11th Edition, 9405.
- 1 2 3 4 5 6 Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- 1 2 3 4 5 6 7 "MSDS of Triacetin". fishersci.ca. Fisher Scientific. Retrieved 2014-06-20.
- 1 2 Woodman, A. L.; Adicoff, A. (1963). "Vapor Pressure of Tiracetin, Triethylene Glycol Dinitrate, and Metriol Trinitrate". Journal of Chemical & Engineering Data. 8 (2): 241. doi:10.1021/je60017a033.
- 1 2 3 4 Triacetin in Linstrom, P.J.; Mallard, W.G. (eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69. National Institute of Standards and Technology, Gaithersburg MD. http://webbook.nist.gov (retrieved 2014-06-20)
- ↑ Kong, P. S., Aroua, M. K., Daud, W. M. A. W., Lee, H. V., Cognet, P., & Peres, Y. (2016). Catalytic role of solid acid catalysts in glycerol acetylation for the production of bio-additives: a review. RSC Advances, 6(73), 68885-68905. doi:10.1039/C6RA10686B
- ↑ "Triacetin". texas-chem.com. Chemical and Filtration Products of Texas. Retrieved 2014-06-20.
- ↑ "Triacetin". drugtopics.modernmedicine.com. Advanstar Communications, Inc. Retrieved 2014-06-20.
- ↑ Gupta, Mayank; Kumar, Naveen "Scope and opportunities of using glycerol as an energy source" Renewable & Sustainable Energy Reviews (2012), 16(7), 4551-4556. doi:10.1016/j.rser.2012.04.001
- ↑ US 6145511
- ↑ Shapira, Jacob; Mandel, Adrian D.; Quattrone, Phillip D.; Bell, Nancie L. (1968). "Current Research On Regenerative Systems" (PDF). casi.ntrs.nasa.gov. Tokyo: Committee On Space Research, Eleventh Annual Meeting. Retrieved 2014-06-20.
- ↑ "Glycerin and Glycerides". www.fda.gov. U.S. Food and Drug Administration. Retrieved 2014-06-20.
- ↑ Final report on the safety assessment of triacetin
