Glucose oxidase
| Glucose oxidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
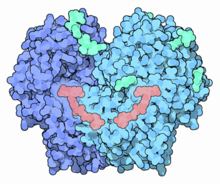 Structure of glucose oxidase dimer (dark and light blue) complexed with FAD (salmon) and glycans (aquamarine) from Penicillium amagasakiense.[1] | |||||||||
| Identifiers | |||||||||
| EC number | 1.1.3.4 | ||||||||
| CAS number | 9001-37-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
The glucose oxidase enzyme (GOx) also known as notatin (EC number 1.1.3.4) is an oxido-reductase that catalyses the oxidation of glucose to hydrogen peroxide and D-glucono-δ-lactone. This enzyme is produced by certain species of fungi and insects and displays antibacterial activity when oxygen and glucose are present.[2]

Glucose oxidase is widely used for the determination of free glucose in body fluids (diagnostics), in vegetal raw material, and in the food industry. It also has many applications in biotechnologies, typically enzyme assays for biochemistry including biosensors in nanotechnologies.[3][4] It is often extracted from Aspergillus niger.
Function
Glucose oxidase is synthesized in several species of fungi and insects where it is used to produce hydrogen peroxide which in turn kills bacteria.[2]
Notatin, extracted from antibacterial cultures of Penicillium notatum, was originally named Penicillin A, but was renamed to avoid confusion with penicillin.[5] Notatin was shown to be identical to Penicillin B and glucose oxidase, enzymes extracted from other molds besides P. notatum;[6] it is now generally known as glucose oxidase.[7]
Early experiments showed that notatin exhibits in vitro antibacterial activity (in the presence of glucose) due to hydrogen peroxide formation.[5][8] In vivo tests showed that notatin was not effective in protecting rodents from Streptococcus haemolyticus, Staphylococcus aureus, or salmonella, and caused severe tissue damage at some doses.[8]
Glucose oxidase is also produced by the hypopharyngeal glands of honeybee workers and deposited into honey where it acts as a natural preservative. GOx at the surface of the honey reduces atmospheric O2 to hydrogen peroxide (H2O2), which acts as an antimicrobial barrier.[9]
Structure
GOx is a dimeric protein, the 3D structure of which has been elucidated. The active site where glucose binds is in a deep pocket. The enzyme, like many proteins that act outside of cells, is covered with carbohydrate chains.
Mechanism
At pH 7, glucose exists in solution in cyclic hemiacetal form as 63.6% β-D-glucopyranose and 36.4% α-D-glucopyranose, the proportion of linear and furanose form being negligible. The glucose oxidase binds specifically to β-D-glucopyranose and does not act on α-D-glucose. It is able to oxidise all of the glucose in solution because the equilibrium between the α and β anomers is driven towards the β side as it is consumed in the reaction.[3]
Glucose oxidase catalyzes the oxidation of β-D-glucose into D-glucono-1,5-lactone, which then hydrolyzes to gluconic acid.
In order to work as a catalyst, GOx requires a cofactor, flavin adenine dinucleotide (FAD). FAD is a common component in biological oxidation-reduction (redox reactions). Redox reactions involve a gain or loss of electrons from a molecule. In the GOx-catalyzed redox reaction, FAD works as the initial electron acceptor and is reduced to FADH2. Then FADH2 is oxidized by the final electron acceptor, molecular oxygen (O2), which can do so because it has a higher reduction potential. O2 is then reduced to hydrogen peroxide (H2O2).
Applications
Glucose oxidase is widely used coupled to peroxidase reaction that visualizes colorimetrically the formed H2O2, for the determination of free glucose in sera or blood plasma for diagnostics, using spectrometric assays manually or with automated procedures, and even point of use rapid assays.[3][7]
Similar assays allows to monitor glucose levels in fermentation, bioreactors, and to control glucose in vegetal raw material and food products. In the glucose oxidase assay, the glucose is first oxidized by glucose oxidase to produce gluconate and hydrogen peroxide. The hydrogen peroxide is then oxidatively coupled with a chromogen to produce a colored compound which may be measured spectroscopically. For example, hydrogen peroxide together with 4 amino-antipyrene (4-AAP) and phenol in the presence of peroxidase yield a red quinoeimine dye that can be measured at 505 nm. The absorbance at 505 nm is proportional to concentration of glucose in the sample.
Enzymatic glucose biosensors use an electrode instead of O2 to take up the electrons needed to oxidize glucose and produce an electronic current in proportion to glucose concentration.[10] This is the technology behind the disposable glucose sensor strips used by diabetics to monitor serum glucose levels.[11]
In manufacturing, GOx is used as an additive thanks to its oxidizing effects: it prompts for stronger dough in bakery, replacing oxidants such as bromate. It also helps remove oxygen from food packaging, or D-glucose from egg white to prevent browning.
Clinical trials
A nasal spray from a bag-on-valve device that mixes glucose oxidase with glucose is undergoing clinical trials for the prevention and treatment of the common cold.[12][13][14]
See also
References
- ↑ PDB: 1gpe; Goodsell D (May 2006). "Molecule of the Month: Glucose Oxidase". RCSB Protein Data Bank. doi:10.2210/rcsb_pdb/mom_2006_5.
- 1 2 Wong CM, Wong KH, Chen XD (Apr 2008). "Glucose oxidase: natural occurrence, function, properties and industrial applications". Applied Microbiology and Biotechnology. 78 (6). doi:10.1007/s00253-008-1407-4. PMID 18330562.
- 1 2 3 "Glucose Oxidase Technical sheet" (PDF). Interchim.
- ↑ Ghoshdastider U, Xu R, Trzaskowski B, Mlynarczyk K, Miszta P, Viswanathan S, Renugopalakrishnan V, Filipek S (2015). "Molecular Effects of Encapsulation of Glucose Oxidase Dimer by Graphene". RSC Advances. 5: 13570–8. doi:10.1039/C4RA16852F.
- 1 2 Coulthard CE, Michaelis R, Short WF, Sykes G (1945). "Notatin: an anti-bacterial glucose-aerodehydrogenase from Penicillium notatum Westling and Penicillium resticulosum sp. nov". The Biochemical Journal. 39 (1): 24–36. doi:10.1042/bj0390024. PMC 1258144
 . PMID 16747849.
. PMID 16747849. - ↑ Keilin D, Hartree EF (Jan 1952). "Specificity of glucose oxidase (notatin)". The Biochemical Journal. 50 (3): 331–41. doi:10.1042/bj0500331. PMC 1197657
 . PMID 14915954.
. PMID 14915954. - 1 2 J ulio Raba J, Mottola HA (1995). "Glucose Oxidase as an Analytical Reagent" (PDF). Critical Reviews in Analytical Chemistry. 25 (1): 1–42. doi:10.1080/10408349508050556.
- 1 2 Broom WA, Coulthard CE, Gurd MR, Sharpe ME (Dec 1946). "Some pharmacological and chemotherapeutic properties of notatin". British Journal of Pharmacology and Chemotherapy. 1 (4): 225–233. doi:10.1111/j.1476-5381.1946.tb00041.x. PMC 1509745
 . PMID 19108091.
. PMID 19108091. - ↑ Bucekova M, Valachova I, Kohutova L, Prochazka E, Klaudiny J, Majtan J (Aug 2014). "Honeybee glucose oxidase--its expression in honeybee workers and comparative analyses of its content and H2O2-mediated antibacterial activity in natural honeys". Die Naturwissenschaften. 101 (8). doi:10.1007/s00114-014-1205-z. PMID 24969731.
- ↑ Blanford CF (Dec 2013). "The birth of protein electrochemistry". Chemical Communications. Royal Society of Chemistry. 49 (95): 11130–11132. doi:10.1039/C3CC46060F. PMID 24153438.
- ↑ Cass AE, Davis G, Francis GD, Hill HA, Aston WJ, Higgins IJ, Plotkin EV, Scott LD, Turner AP (Apr 1984). "Ferrocene-mediated enzyme electrode for amperometric determination of glucose". Analytical Chemistry. American Chemical Society. 56 (4): 667–671. doi:10.1021/ac00268a018. PMID 6721151.
- ↑ Clinical trial number NCT01883427 for "Nasal Spray With Glucose Oxidase Preventing Common Cold in Pre-school Children" at ClinicalTrials.gov
- ↑ Clinical trial number NCT01883440 for "Glucose Oxidase as Treatment Against Common Cold" at ClinicalTrials.gov
- ↑ Clinical trial number NCT01883453 for "A Nasal Spray With Glucose Oxidase as a Treatment of Common Cold" at ClinicalTrials.gov
External links
- "Glucose Oxidase: A much used and much loved enzyme in biosensors" at University of Paisley
- Glucose Oxidase at the US National Library of Medicine Medical Subject Headings (MeSH)