Gestational hypertension
| Pregnancy-induced hypertension | |
|---|---|
 | |
| Micrograph showing hypertrophic decidual vasculopathy, the histomorphologic correlate of gestational hypertension. H&E stain. | |
| Classification and external resources | |
| Specialty | obstetrics |
| ICD-10 | O13-O14 |
| ICD-9-CM | 642 |
| DiseasesDB | 5208 |
| MedlinePlus | 000898 |
| eMedicine | med/3250 |
| MeSH | D046110 |
Gestational hypertension or pregnancy-induced hypertension (PIH) is the development of new hypertension in a pregnant woman after 20 weeks gestation without the presence of protein in the urine or other signs of preeclampsia.[1] Hypertension is defined as having a blood pressure greater than 140/90 mm Hg.[1]
Conditions
There exist several hypertensive states of pregnancy:
- Gestational hypertension
- Gestational hypertension is usually defined as having a blood pressure higher than 140/90 measured on two separate occasions, more than 6 hours apart, without the presence of protein in the urine and diagnosed after 20 weeks of gestation.[2]
- Preeclampsia
- Pre-eclampsia is gestational hypertension plus proteinuria (>300 mg of protein in a 24-hour urine sample). Severe preeclampsia involves a blood pressure greater than 160/110, with additional medical signs and symptoms. HELLP syndrome is a type of preeclampsia. It is a combination of three medical conditions: hemolytic anemia, elevated liver enzymes and low platelet count.
- Eclampsia
- This is when tonic-clonic seizures appear in a pregnant woman with high blood pressure and proteinuria.
Pre-eclampsia and eclampsia are sometimes treated as components of a common syndrome.[3]
Risk factors
Maternal causes
- Obesity
- Age 35 years or more.
- Past history of diabetes mellitus, hypertension and renal disease.
- Adolescent pregnancy.
- New paternity.
- Thrombophilias (anti-phospholoipid syndrome, protein C/S deficiency, factor V Leiden)
- Having donated a kidney.[4]
Pregnancy
- Multiple gestation ( twins or triplets, etc.)
- Placental abnormalities:
- 1. Hyperplacentosis: Excessive exposure to chorionic villi.
- 2. Placental ischemia.
Family history
- Family history of pre-eclampsia.
- African American race
Treatment
There is no specific treatment, but is monitored closely to rapidly identify pre-eclampsia and its life-threatening complications (HELLP syndrome and eclampsia).
Drug treatment options are limited, as many antihypertensives may negatively affect the fetus. Methyldopa, hydralazine, and labetalol are most commonly used for severe pregnancy hypertension.
The fetus is at increased risk for a variety of life-threatening conditions, including pulmonary hypoplasia (immature lungs). If the dangerous complications appear after the fetus has reached a point of viability, even though still immature, then an early delivery may be warranted to save the lives of both mother and baby. An appropriate plan for labor and delivery includes selection of a hospital with provisions for advanced life support of newborn babies.
Evolutionary considerations
Humans
Gestational hypertension is one of the most common disorders seen in human pregnancies.[5] Though relatively benign on its own, in roughly half of the cases of gestational hypertension the disorder progresses into preeclampsia, a dangerous condition that can prove fatal to expectant mothers.[6] However, gestational hypertension is a condition that is fairly rare to see in other animals. For years, it has been the belief of the scientific community that gestational hypertension and preeclampsia were relatively unique to humans, although there has been some recent evidence that other primates can also suffer from similar conditions, albeit due to different underlying mechanisms.[5] The underlying cause of gestational hypertension in humans is commonly believed to be an improperly implanted placenta. Humans have evolved to have a very invasive placenta to facilitate better oxygen transfer from the mother to the fetus, to support the growth of its large brain.[7]
Origins of the placenta
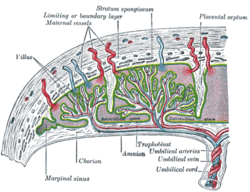
The origins of gestational hypertension may lie with the development of humans’ hemochorial placenta. A hemochorial placenta optimizes the amount of oxygen and nutrients that can be absorbed into the fetal blood supply, while at the same time ensuring rapid diffusion of wastes away from the fetus. This hemochorial placenta differs from lower primates’ epitheliochorial placentae in the way that it allows the fetal tissues to interact directly with the mother’s blood. The hemochorial placenta thereby promotes more rapid diffusion to and from the fetal blood supply.[8]
In animals with epitheliochorial placentae such as horses and pigs, the greatest resistance to maternal blood flow in the vascular system was found within the placenta. However, in animals with hemochorial placental structures such as rodents and primates, the vascular resistance in the placenta was low, leading scientists to the conclusion that the greatest resistance to maternal blood flow is found elsewhere in the maternal vascular system.[9] The high vascular resistance outside of the placenta leads to higher maternal blood pressure throughout the body.
The fetal cells that implant into the uterine wall are known as the trophoblast. The hemochorial placenta bathes the fetal trophoblast in maternal blood by forming lacunae, or lakes, of the mother’s blood that surround fetal tissue. The lacunae are filled by the spiral arteries, which means that the mother’s blood pressure is the driving force behind the introduction of new blood, which contains both oxygen and food for the fetus, to the system.[10] It is thought that humans need the increased diffusion provided by the hemochorial placenta in order to grow the large brains compared to their body size that distinguish them from other primates.[11]
Incorrect placental implantation
It is thought that “failings” in normal hemochorial placental structure lead to preeclampsia and gestational hypertension.[12] The human placenta implants “earlier, deeper, and more extensively” into the uterine wall, which can potentially lead to many problems that are found in human pregnancies, but not as much in other animals. Miscarriage and preeclampsia are both very rare in other species, but are two of the most common pregnancy-related diseases in humans.[13] The genetic roots of gestational hypertension and preeclampsia are certain, as women with a family history of the condition are three times more likely to suffer from it when they are pregnant.[14]
One of the potential causes of gestational hypertension and preeclampsia is when the trophoblast does not invade far enough into the uterine lining.[15] When the fetus’ trophoblast does not fully extend into the uterine wall, the spiral arteries do not become fully converted into low-resistance channels.[13] It has been found that this incomplete conversion of spiral arteries increases the resistance to uterine blood flow during pregnancy, and that this occurrence was associated with gestational hypertension.[16] One potential cause of this incomplete breach of the spiral arteries that leads to gestational hypertension is a mistaken immune response by the maternal tissue, reaction to the alien fetal tissue.[17] Therefore, it is clear that the complication of gestational hypertension has roots in the early implantation of the fetus in the uterine wall, an implantation technique that is unique to humans.
The highly invasive placenta that is found in humans is thought to be linked to humans’ high circulating levels of the hormones CG and hCG. It has been shown that the higher the levels of these hormones, the deeper the trophoblast’s invasion into the uterine wall. Instances of gestational hypertension and preeclampsia have been shown to occur when the invasion of the uterine wall is not deep enough, because of lower CG and hCG levels in the mother.[18]
Evolutionary tradeoff
Despite these risks for gestational hypertension, the hemochorial placenta has been favored because of its advantages in the way that it aids in diffusion from mother to fetus later in pregnancy. The bipedal posture that has allowed humans to walk upright has also led to a reduced cardiac output, and it has been suggested that this is what necessitated humans’ aggressive early placental structures.[19] Increased maternal blood pressure can attempt to make up for lower cardiac output, ensuring that the fetus’s growing brain receives enough oxygen and nutrients.[18] The benefits of being able to walk upright and run on land have outweighed the disadvantages that come from bipedalism, including the placental diseases of pregnancy, such as gestational hypertension. Similarly, the advantages of having a large brain size have outweighed the deleterious effects of having a placenta that does not always convert the spiral arteries effectively, leaving humans vulnerable to contracting gestational hypertension. It is speculated that this was not the case with Neanderthals, and that they died out because their cranial capacity increased too much, and their placentae were not equipped to handle the fetal brain development, leading to widespread preeclampsia and maternal and fetal death.[20]
Gestational hypertension in the early stages of pregnancy has been shown to improve the health of the child both in its first year of life, and its later life.[21] However, when the disease develops later in the pregnancy, or turns into preeclampsia, there begin to be detrimental health effects for the fetus, including low birth-weight.[6] It has been proposed that fetal genes designed to increase the mother’s blood pressure are so beneficial that they outweigh the potential negative effects that can come from preeclampsia.[21] It has also been suggested that gestational hypertension and preeclampsia have remained active traits due to the cultural capacity of humans, and the tendency for midwives or helpers to aid in delivering babies.[22]
Relevance of evolutionary history
It is the goal of evolutionary medicine to find treatments for diseases that are informed by the evolutionary history of a disease. It has been suggested that gestational hypertension is linked to insulin resistance during pregnancy.[23] Both the increase in blood sugar that can lead to gestational diabetes and the increase in blood pressure that can lead to gestational hypertension are mechanisms that mean to optimize the amount of nutrients that can be passed from maternal tissue to fetal tissue. It has been suggested that techniques used to combat insulin insensitivity might also prove beneficial to those suffering from gestational hypertension.[23] Measures to avoid insulin resistance include avoiding obesity before pregnancy, minimizing weight gain during pregnancy, eating foods with low glycemic indexes, and exercising.[23]
References
- 1 2 "40". Williams obstetrics (24th ed.). McGraw-Hill Professional. 2014. ISBN 9780071798938.
- ↑ Lo, JO; Mission, JF; Caughey, AB (April 2013). "Hypertensive disease of pregnancy and maternal mortality.". Current opinion in obstetrics & gynecology. 25 (2): 124–32. doi:10.1097/gco.0b013e32835e0ef5. PMID 23403779.
- ↑ "preeclampsia/eclampsia" at Dorland's Medical Dictionary
- ↑ Garg, Amit X.; Nevis, Immaculate F.; McArthur, Eric; Sontrop, Jessica M.; Koval, John J.; Lam, Ngan N.; Hildebrand, Ainslie M.; Reese, Peter P.; Storsley, Leroy; Gill, John S.; Segev, Dorry L.; Habbous, Steven; Bugeja, Ann; Knoll, Greg A.; Dipchand, Christine; Monroy-Cuadros, Mauricio; Lentine, Krista L. (2014). "Gestational Hypertension and Preeclampsia in Living Kidney Donors". New England Journal of Medicine. 372: 141114133004008. doi:10.1056/NEJMoa1408932. ISSN 0028-4793.
- 1 2 Abrams, Elizabeth, and Julienne Rutherford. "Framing Postpartum Hemorrhage as a Consequence of Human Placental Biology: An Evolutionary and Comparative Perspective." Am Anthropol 113.3 (2011): 417-30.
- 1 2 Barton, J. "Mild Gestational Hypertension Remote from Term: Progression and Outcome." American Journal of Obstetrics and Gynecology 184.5 (2001): 979-83.
- ↑ Rosenberg, Karen R., and Wenda R. Trevathan. "An Anthropological Perspective on the Evolutionary Context of Preeclampsia in Humans." Journal of Reproductive Immunology 76.1-2 (2007): 91-97.
- ↑ Campbell, Bernard Grant. "Reproduction and the Placenta." Human Evolution: An Introduction to Man's Adaptations. New York: Aldine De Gruyter, 1998. 317-20.
- ↑ Moll, Waldemar, and Wolfgang Kunzel. "The Blood Pressure in Arteries Entering the Placentae of Guinea Pigs, Rats, Rabbits, and Sheep." Pflugers Archiv European Journal of Physiology 338.2 (1973): 125-31.
- ↑ Ahokas, R, McKinney, E, Glob. libr. women's med., (ISSN 1756-2228) 2008; doi:10.3843/GLOWM.10101
- ↑ Martin RD (2003) Human reproduction: a comparative background for medical hypothesis. J Reprod Immunol 59,111–135.
- ↑ Cross, JC. "The Genetics of Pre-eclampsia: A Feto-placental or Maternal Problem?" Clinical Genetics 64.2 (2003): 96-103.
- 1 2 Jauniaux, E., L. Poston, and G. J. Burton. "Placental-related Diseases of Pregnancy: Involvement of Oxidative Stress and Implications in Human Evolution." Human Reproduction Update 12.6 (2006): 747-55.
- ↑ Duckitt, K. "Risk Factors for Pre-eclampsia at Antenatal Booking: Systematic Review of Controlled Studies." Bmj 330.7491 (2005): 565.
- ↑ Norwitz, Errol R. "Defective Implantation and Placentation: Laying the Blueprint for Pregnancy Complications." Reproductive BioMedicine Online 13.4 (2006): 591-99.
- ↑ Olofsson, P., R. Laurini, and K. Marsal. "A High Uterine Artery Pulsatility Index Reflects a Defective Development of Placental Bed Spiral Arteries in Pregnancies Complicated by Hypertension and Fetal Growth Retardation." European Journal of Obstetrics & Gynecology and Reproductive Biology 49.3 (1993): 161-68.
- ↑ Robertson, W. B., I. Bronsens, and G. Dixon. "Maternal Uterine Vascular Lesions in the Hypertensive Complications of Pregnancy." Perspect Nephrol Hypertens. 5 (1976): 115-27.
- 1 2 Cole, Laurence A. "HCG and Hyperglycosylated HCG in the Establishment and Evolution of Hemochorial Placentation." Journal of Reproductive Immunology 82.2 (2009): 112-18
- ↑ Rockwell LC, Vargas E and Moore L (2003) Human physiological adaptation to pregnancy: inter- and intraspecific perspectives. Am J Hum Biol 15,330–341.
- ↑ Chaline, Jean. "Increased Cranial Capacity in Hominid Evolution and Preeclampsia." Journal of Reproductive Immunology 59.2 (2003): 137-52.
- 1 2 Hollegaard B, Byars SG, Lykke J, Boomsma JJ (2013). "Parent-Offspring Conflict and the Persistence of Pregnancy-Induced Hypertension in Modern Humans". PLoS ONE. 8 (2): e56821. doi:10.1371/journal.pone.0056821.
- ↑ Rosenberg Karen R.; Trevathan Wenda R. (2007). "An Anthropological Perspective on the Evolutionary Context of Preeclampsia in Humans". Journal of Reproductive Immunology. 76 (1-2): 91–97. doi:10.1016/j.jri.2007.03.011.
- 1 2 3 Solomon Caren G.; Seely Ellen W. (2001). "Brief Review: Hypertension in Pregnancy A Manifestation of the Insulin Resistance Syndrome?". Hypertension. 37: 232–39. doi:10.1161/01.hyp.37.2.232.