GelRed
 | |
| Names | |
|---|---|
| IUPAC name
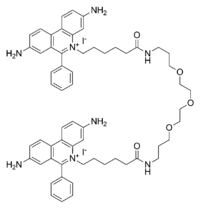
5,5'-(6,22-dioxo-11,14,17-trioxa-7,21-diazaheptacosane-1,27-diyl)bis(3,8-diamino-6-phenylphenanthridin-5-ium) iodide | |
| Other names | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| |
| |
| Properties | |
| C60H72I2N8O5 | |
| Molar mass | 1239.07 g/mol |
| Hazards | |
| Safety data sheet | 10,000X in water, Biotium Inc. |
| R-phrases | R25 R36/37/38 |
| S-phrases | S22 S24/25 S26 S36/37/39 S45 S53 |
| NFPA 704 | |
| Flash point | > 100 °C (212 °F; 373 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
GelRed is an intercalating nucleic acid stain used in molecular biology for agarose gel electrophoresis. GelRed is structurally closely related to ethidium bromide and consists of two ethidium subunits that are bridged by a linear spacer.[1][2]
Its fluorophore, and therefore its optical properties, are essentially identical to those of ethidium bromide. When exposed to ultraviolet light, it will fluoresce with an orange color that strongly intensifies after binding to DNA.[3] The substance is marketed as a less toxic and more sensitive alternative to ethidium bromide.[3] GelRed is sold as a solution in DMSO or, more recently, in water.[3]
See also
- Ethidium bromide
- GelGreen
- SYBR Green I
- Agarose gel electrophoresis and gel electrophoresis of nucleic acids
- Phenanthridine
References
- 1 2 3 US application 2010323453, Mao, Fei & Leung, Wai-Yee, "Methods of Using Dyes in Association with Nucleic Acid Staining or Detection and Associated Technology"
- ↑ GelRed & GelGreen (PDF), Biotium Inc., August 21, 2012, retrieved December 4, 2012
- 1 2 3 GelRed and GelGreen: Environmentally safe and ultra-sensitive nucleic acid gel stains for replacing EtBr, Biotium Inc., retrieved December 4, 2012
This article is issued from Wikipedia - version of the 6/6/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
