Gcn2
| Serine/threonine-protein kinase GCN2 | |
|---|---|
 Crystal structure of GCN2 | |
| Identifiers | |
| Symbol | GCN2 |
| Alt. symbols | AAS1 |
| Entrez | 851877 |
| PDB | 1zyc |
| UniProt | P15442 |
GCN2 (general control nonderepressible 2) is a serine/threonine-protein kinase that senses amino acid deficiency through binding to uncharged transfer RNA (tRNA). It plays a key role in modulating amino acid metabolism as a response to nutrient deprivation.
Introduction
GCN2 is the only known eukaryotic initiation factor 2α kinase (eIF2α) in Saccharomyces cerevisiae.[1] It inactivates eIF2α by phosphorylation at Serine 51 under conditions of amino acid deprivation, resulting in repression of general protein synthesis whilst allowing selected mRNA such as GCN4 to be translated due to regions upstream of the coding sequence. Elevated levels of GCN4 stimulate the expression of amino acid biosynthetic genes, which code for enzymes required to synthesize all 20 major amino acids.
Structure
Protein kinase GCN2 is a multidomain protein and its C-terminus contains a region homologous to histidyl-tRNA synthetase (HisRS) next to the kinase catalytic moiety.[2] This HisRS-like region forms a dimer and dimerization is required for GCN2 function. The crucial contribution to GCN2 function is the promotion of tRNA binding and the stimulation of the kinase domain via physical interaction.
Binding of uncharged tRNA to this synthetase-like domain induces a conformational change in which the GCN2 domains rotate 180° normal to the dimerization surface and thereby transpose from their antiparallel to a parallel orientation. Subsequently the autoinhibited form of GCN2 is activated.[3]
GCN2 autoinhibition results from a conformation that restricts ATP binding. ATP binding induces autophosphorylation of an activation loop which leads to maximal GCN2 kinase activity.[4]
Function
Regulation of translation
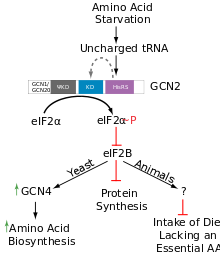
GCN2 inhibits general translation by phosphorylation of eIF-2α at serine 51 within 15 min of amino acid deprivation, which then subsequently increases the affinity for the guanine exchange factor eIF2B to sequester eIF-2α leading to reduced formation of the ternary complex (TC) consisting of eIF2, GTP and the initiator Met-tRNA required for translation initiation. eIF2 containing a phosphorylated alpha subunit shows an increased affinity for its only GEF, eIF2B, but eIF2B is only able to exchange GDP with GTP from unphosphorylated eIF2. So the recycling of eIF2, needed for TC formation, is inhibited by phosphorylation of eIF-2α, which in the end leads to a reduction of global translation rates.
An opposing effect of the reduced availability of TC is the induction of GCN4 expression by translational regulation. Four short ORF's exist in the leader of the GCN4 mRNA. 40S Ribosomal Subunits scanning the mRNA from 5' have TC bound and translate the first upstream open reading frame (uORF). Under non-starving condition there is enough ternary complex that the subunits rebind it before they reach uORF 4. Translation is again initiated, uORF2,3 or 4 translated and the 40S Subunits subsequently dissociate from GCN4 mRNA. Under starving conditions there is less TC present. Some of the 40S Subunits are not able to rebind TC before they reach uORF 4 but eventually rebind TC before reaching GCN4 coding sequence. Therefore, the reduction in TC formation resulting from GCN2 activation by amino acid starvation leads to the induction of GCN4 translation. GCN4 is the primary regulator in response to amino acid starvation, termed general amino acid control (GAAC). It acts as a transcription factor and activates several genes required for amino acid synthesis.[5][6][7]
Recently GCN2 has also been implicated in directing eating behavior in mammals by phosphorylating eIF-2α in the anterior Piriform cortex (APC) of the brain. The molecular mechanisms governing this function are not yet known, but a basic zipper transcription factor called ATF4 is a possible candidate.[5] ATF4 is related to GCN4.[1]
Cell cycle control
GCN2 also regulates the cell cycle by delaying entry into S phase upon ultraviolet (UV) radiation and exposure to methyl methanesulfonate (MMS).[8][9] Thereby the cell prevents passing the G1 checkpoint and starting DNA replication when the DNA is damaged. It has been hypothesized, that UV induces nitric oxide synthase activation and NO. production, which leads to the activation of GCN2 and that the cell cycle regulation by GCN2 is independent of eIF2α phosphorylation.[10] Although the causal relationship between GCN2 and cell cycle delay is still under debate, it was suggested that the formation of the pre-replication complex is deferred by GCN2 upon UV-irradiation.[8]
Lipid metabolism
The absence of essential amino acids causes a downregulation of key components of the lipid synthesis such as the fatty acid synthase. Following leucine-deprivation in mammals, GCN2 decreases the expression of lipogenic genes via SREBP-1c.[11] SREBP-1c actions upon genes regulating fatty-acid and triglyceride synthesis and is reduced by leucine deprivation in the liver in a GCN2-dependant manner.
Regulation
In amino acid replete cells, GCN2 is kept inactive via phosphorylation at serine 577, which is thought to depend on the activity of TORC1. Inactivation of TORC1 by Rapamycin affects GCN2 and at least partly by dephosphorylation of serine 577. This leads to activation of GCN2 even in amino acid replete cells, probably by increasing the affinity of GCN2 for uncharged tRNA, so that even basal levels permit tRNA binding.
A second stimulatory input to GCN2 is exerted by a complex of GCN1/GCN20. GCN1/GCN20 shows structural similarity to eEF3, a factor important in the binding of tRNA to ribosomes. The GCN1/GCN20 complex physically interacts with GCN2 by binding to its N-terminus.[12] It is thought that GCN1/GCN20 facilitates the transfer of tRNA from the ribosomal A site to the HisRS-like domain of GCN2.[7] A third way of regulation of this protein is through the conserved protein IMPACT, that acts both in yeast, nematodes and mammals as an inhibitor of GCN2.[13][14][15]
Homologues
There are also GCN2 homologues in Neurospora crassa,[16] C. elegans,[15] Drosophila melanogaster[17][18] and mice.[19] Thus, GCN2 may be the most widespread and founding member of the eIF-2α kinase subfamily.[20]
See also
- Eukaryotic initiation factors
- The three subunits of eIF2:
- Kinases of eIF2
- EIF2AK4
References
- 1 2 Zaborske JM, Narashimhan J, Jiang L, Wek SA, Dittmar KA, Freimoser F, Pan T, Wek RC (Sep 2009). "Genome-wide analysis of tRNA charging and activation of the eIF2 kinase Gcn2p". J Biol Chem. 284 (37): 25254–67. doi:10.1074/jbc.M109.000877. PMC 2757228
 . PMID 19546227.
. PMID 19546227. - ↑ Wek SA, Zhu S, Wek RC (August 1995). "The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids". Mol. Cell. Biol. 15 (8): 4497–506. PMC 230689
 . PMID 7623840.
. PMID 7623840. - ↑ Dey M, Cao C, Sicheri F, Dever TE (March 2007). "Conserved intermolecular salt bridge required for activation of protein kinases PKR, GCN2, and PERK". J. Biol. Chem. 282 (9): 6653–60. doi:10.1074/jbc.M607897200. PMID 17202131.
- ↑ PDB: 1ZYC Padyana AK, Qiu H, Roll-Mecak A, Hinnebusch AG, Burley SK (August 2005). "Structural basis for autoinhibition and mutational activation of eukaryotic initiation factor 2alpha protein kinase GCN2". J. Biol. Chem. 280 (32): 29289–99. doi:10.1074/jbc.M504096200. PMID 15964839.
- 1 2 3 Dever TE, Hinnebusch AG (April 2005). "GCN2 whets the appetite for amino acids". Mol. Cell. 18 (2): 141–2. doi:10.1016/j.molcel.2005.03.023. PMID 15837415.
- ↑ Wek RC (November 1994). "eIF-2 kinases: regulators of general and gene-specific translation initiation". Trends Biochem. Sci. 19 (11): 491–6. doi:10.1016/0968-0004(94)90136-8. PMID 7855893.
- 1 2 Hinnebusch AG (2005). "Translational regulation of GCN4 and the general amino acid control of yeast". Annu. Rev. Microbiol. 59: 407–50. doi:10.1146/annurev.micro.59.031805.133833. PMID 16153175.
- 1 2 Tvegård T, Soltani H, Skjølberg HC, Krohn M, Nilssen EA, Kearsey SE, Grallert B, Boye E (May 2007). "A novel checkpoint mechanism regulating G1/S transition". Genes Dev. 21 (6): 649–54. doi:10.1101/gad.421807. PMC 1820939
 . PMID 17369398.
. PMID 17369398. - ↑ Krohn M, Skjølberg HC, Soltani H, Grallert B, Boye E (December 2008). "The G1-S checkpoint in fission yeast is not a general DNA damage checkpoint". J Cell Sci. 121 (24): 4047–54. doi:10.1242/jcs.035428. PMID 19033384.
- ↑ Wang L, Liu Y, Wu S (January 2010). "The roles of nitric oxide synthase and eIF2alpha kinases in regulation of cell cycle upon UVB-irradiation". Cell Cycle. 9 (1): 38–42. doi:10.4161/cc.9.1.10268. PMC 2834424
 . PMID 20016280.
. PMID 20016280. - ↑ Guo F, Cavener DR (Feb 2007). "The GCN2 eIF2α Kinase Regulates Fatty-Acid Homeostasis in the Liver during Deprivation of an Essential Amino Acid". Cell Metab. 5 (2): 103–14. doi:10.1016/j.cmet.2007.01.001. PMID 17276353.
- ↑ Garca-Barrio M, Dong J, Ufano S, Hinnebusch AG (Apr 2000). "Association of GCN1-GCN20 regulatory complex with the N-terminus of eIF2alpha kinase GCN2 is required for GCN2 activation". EMBO J. 19 (8): 1887–99. doi:10.1093/emboj/19.8.1887. PMC 302013
 . PMID 10775272.
. PMID 10775272. - ↑ Sattlegger, Evelyn; Swanson, Mark J.; Ashcraft, Emily A.; Jennings, Jennifer L.; Fekete, Richard A.; Link, Andrew J.; Hinnebusch, Alan G. (2004-07-16). "YIH1 Is an Actin-binding Protein That Inhibits Protein Kinase GCN2 and Impairs General Amino Acid Control When Overexpressed". Journal of Biological Chemistry. 279 (29): 29952–29962. doi:10.1074/jbc.M404009200. ISSN 0021-9258.
- ↑ Pereira, Cátia M.; Sattlegger, Evelyn; Jiang, Hao-Yuan; Longo, Beatriz M.; Jaqueta, Carolina B.; Hinnebusch, Alan G.; Wek, Ronald C.; Mello, Luiz E. A. M.; Castilho, Beatriz A. (2005-08-05). "IMPACT, a Protein Preferentially Expressed in the Mouse Brain, Binds GCN1 and Inhibits GCN2 Activation". Journal of Biological Chemistry. 280 (31): 28316–28323. doi:10.1074/jbc.M408571200. ISSN 0021-9258.
- 1 2 Ferraz, Rafael C.; Camara, Henrique; De-Souza, Evandro A.; Pinto, Silas; Pinca, Ana Paula F.; Silva, Richard C.; Sato, Vitor N.; Castilho, Beatriz A.; Mori, Marcelo A. (2016-01-01). "IMPACT is a GCN2 inhibitor that limits lifespan in Caenorhabditis elegans". BMC Biology. 14: 87. doi:10.1186/s12915-016-0301-2. ISSN 1741-7007.
- ↑ Sattlegger E, Hinnebusch AG, Barthelmess IB (Aug 1998). "cpc-3, the Neurospora crassa homologue of yeast GCN2, encodes a polypeptide with juxtaposed eIF2alpha kinase and histidyl-tRNA synthetase-related domains required for general amino acid control". J Biol Chem. 273 (32): 20404–16. doi:10.1074/jbc.273.32.20404. PMID 9685394.
- ↑ Santoyo J, Alcalde J, Méndez R, Pulido D, de Haro C (May 1997). "Cloning and Characterization of a cDNA Encoding a Protein Synthesis Initiation Factor-2α (eIF-2α) Kinase from Drosophila melanogaster". J Biol Chem. 272 (19): 12544–50. doi:10.1074/jbc.272.19.12544. PMID 9139706.
- ↑ Olsen DS, Jordan B, Chen D, Wek RC, Cavender DR (Jul 1998). "Isolation of the gene encoding the Drosophila melanogaster homolog of the Saccharomyces cerevisiae GCN2 eIF-2alpha kinase". Genetics. 149 (3): 1495–509. PMC 1460234
 . PMID 9649537.
. PMID 9649537. - ↑ Sood R, Porter AC, Olsen DA, Cavener DR, Wek RC (Feb 2000). "A mammalian homologue of GCN2 protein kinase important for translational control by phosphorylation of eukaryotic initiation factor-2alpha". Genetics. 154 (2): 787–801. PMC 1460965
 . PMID 10655230.
. PMID 10655230. - ↑ Dever TE (Oct 1999). "Translation initiation: adept at adapting". Trends Biochem Sci. 24 (10): 398–403. doi:10.1016/s0968-0004(99)01457-7. PMID 10500305.