Furanocoumarin
The furanocoumarins, or furocoumarins, are a class of organic chemical compounds produced by a variety of plants. They are biosynthesized partly through the phenylpropanoid pathway and the mevalonate pathway, which is biosynthesized by a coupling of dimethylallyl pyrophosphate (DMAPP) and 7-hydroxycoumarin (umbelliferone).
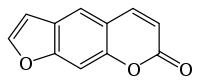
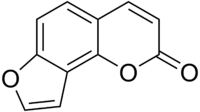
The chemical structure of furanocoumarins consists of a furan ring fused with coumarin. The furan may be fused in different ways producing several isomers. The compounds that form the core structure of the two most common isomers are psoralen and angelicin. Derivatives of these two core structures are referred to respectively as linear and angular furanocoumarins.[1]
Many furanocoumarins are toxic and are produced by plants as a defense mechanism against various types of predators ranging from insects to mammals.[2] This class of phytochemical is responsible for the phytophotodermatitis seen in exposure to the juices of the wild parsnip and the giant hogweed. There are also reports of these chemicals in the species Dictamnus albus.
Furanocoumarins have other biological effects as well. For example, in humans, bergamottin and 6',7'-dihydroxybergamottin are responsible for the "grapefruit juice effect", in which these furanocoumarins affect the metabolism of certain drugs.[3]
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "furanocoumarins". IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "furocoumarins".
- ↑ Berenbaum, May (May 14, 2010). "Furanocoumarins as potent chemical defenses".
- ↑ Kakar, SM; Paine, MF; Stewart, PW; Watkins, PB (2004). "6',7'-Dihydroxybergamottin contributes to the grapefruit juice effect". Clinical Pharmacology and Therapeutics. 75 (6): 569–579. doi:10.1016/j.clpt.2004.02.007. PMID 15179411.