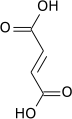
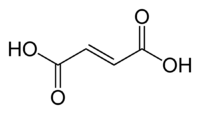
Fumaric acid
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2E)-But-2-enedioic acid | |
| Other names
Fumaric acid trans-1,2-Ethylenedicarboxylic acid 2-Butenedioic acid trans-butenedioic acid Allomaleic acid Boletic acid Donitic acid Lichenic acid | |
| Identifiers | |
| 110-17-8 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:18012 |
| ChEMBL | ChEMBL503160 |
| ChemSpider | 10197150 |
| DrugBank | DB04299 |
| ECHA InfoCard | 100.003.404 |
| EC Number | 203-743-0 |
| E number | E297 (preservatives) |
| KEGG | C00122 |
| UNII | 88XHZ13131 |
| |
| |
| Properties | |
| C4H4O4 | |
| Molar mass | 116.07 g·mol−1 |
| Appearance | White solid |
| Density | 1.635 g/cm3 |
| Melting point | 287 °C (549 °F; 560 K) (decomp)[1] |
| 4.9 g/L at 20°C[2] | |
| Acidity (pKa) | pka1 = 3.03, pka2 = 4.44 |
| Pharmacology | |
| D05AX01 (WHO) | |
| Hazards | |
| EU classification (DSD) |
Irritant (Xi) |
| R-phrases | R36 |
| S-phrases | (S2)-S26 |
| NFPA 704 | |
| Related compounds | |
| Related carboxylic acids |
maleic acid succinic acid crotonic acid |
| Related compounds |
fumaryl chloride fumaronitrile dimethyl fumarate iron(II) fumarate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Fumaric acid or trans-butenedioic acid is the chemical compound with the formula HO2CCH=CHCO2H. This white crystalline compound is one of two isomeric unsaturated dicarboxylic acids, the other being maleic acid. In fumaric acid the carboxylic acid groups are trans (E) and in maleic acid they are cis (Z). Fumaric acid has a fruit-like taste. The salts and esters are known as fumarates.
Chemistry
Fumaric acid was first prepared from succinic acid.[3] A traditional synthesis involves oxidation of furfural (from the processing of maize) using chlorate in the presence of a vanadium-based catalyst.[4] Currently, industrial synthesis of fumaric acid is mostly based on catalytic isomerisation of maleic acid in aqueous solutions at low pH. Maleic acid is accessible in large volumes as a hydrolysis product of maleic anhydride, produced by catalytic oxidation of benzene or butane.[5]
The chemical properties of fumaric acid can be anticipated from its component functional groups. This weak acid forms a diester, it undergoes additions across the double bond, and it is an excellent dienophile.
Fumaric acid does not combust in a bomb calorimeter under conditions where maleic acid deflagrates smoothly. For teaching experiments designed to measure the difference in energy between the cis- and trans- isomers, a measured quantity of carbon can be ground with the subject compound and the enthalpy of combustion computed by difference.
Biology
Fumaric acid is found in fumitory (Fumaria officinalis), bolete mushrooms (specifically Boletus fomentarius var. pseudo-igniarius), lichen, and Iceland moss.
Fumarate is an intermediate in the citric acid cycle used by cells to produce energy in the form of adenosine triphosphate (ATP) from food. It is formed by the oxidation of succinate by the enzyme succinate dehydrogenase. Fumarate is then converted by the enzyme fumarase to malate. Human skin naturally produces fumaric acid when exposed to sunlight.[6]
Fumarate is also a product of the urea cycle.
Uses
Food
Fumaric acid has been used as a food acidulant since 1946. It is approved for use as a food additive in the EU,[7] USA[8] and Australia and New Zealand.[9] As a food additive, it is used as an acidity regulator and can be denoted by the E number E297. It is generally used in beverages and baking powders for which requirements are placed on purity. It is generally used as a substitute for tartaric acid and occasionally in place of citric acid, at a rate of 1 g of fumaric acid to every ~1.5 g of citric acid, in order to add sourness, similarly to the way malic acid is used. As well as being a component of some artificial vinegar flavors, such as "Salt and Vinegar" flavored potato chips,[10] it is also used as a coagulant in stovetop pudding mixes.
Medicine
In patients with relapsing-remitting multiple sclerosis, dimethyl fumarate (BG-12, Biogen) significantly reduced relapse and disability progression in a phase 3 trial. It activates the Nrf2 antioxidant response pathway, the primary cellular defense against the cytotoxic effects of oxidative stress.[11]
Psoriasis
Various Fumaric acid esters (FAES) have specific immunomodulating (respectively moderate immunosuppression) properties which are responsible for certain effects in the treatment of psoriasis. In the Netherlands, Germany, Austria and the Benelux countries substances derived from fumaric acid have been used for half a century for this purpose.[12] Although it seems that a certain effectiveness exists (and many studies have confirmed the effect), more statistically powerful studies are necessary to provide better evidence of the effectiveness of fumaric acid esters in treatment of psoriasis (e.g. more accurate in terms of the dermatological nosology of this autoimmune disease). Such studies should in the future help specify which psoriasis variants (in terms of the course, the immunological profile of the patient etc.) are suited to be treated with fumaric acid esters.
Fumaric acid is naturally present in the herb called Fumaria officinalis. The top of this herb is used to treat various skin disorders including psoriasis. Baths with this herb have been used for several centuries.[13] At present, these baths (after consultation with a general practitioner or dermatologist) are used as an adjunct therapy to basic treatment.
Other uses
Fumaric acid is used in the manufacture of polyester resins and polyhydric alcohols and as a mordant for dyes. Since the early 21st century, it has been used to synthesize one of the first metal-organic frameworks presenting commercial applications thanks to its remarkable adsorption and mechanical properties, combined with a low toxicity compared to other well-studied MOFs.[14]
Safety
Fumaric acid converts to the irritant maleic anhydride, upon partial combustion.
It is "practically non-toxic" but high doses are probably nephrotoxic after long-term use.[15]
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
TCA Cycle edit
- ↑ The interactive pathway map can be edited at WikiPathways: "TCACycle_WP78".
See also
- Dermatology
- Photosynthesis
- Maleic acid, the cis-isomer of fumaric acid
References
- ↑ Fumaric acid, PubChem
- ↑ Record in the GESTIS Substance Database of the IFA
- ↑ Volhard, J. "Darstellung von Maleïnsäureanhydrid" Justus Liebig's Annalen der Chemie 1892, volume 268, page 255-6. doi:10.1002/jlac.18922680108
- ↑ Milas, N. A. "Fumaric Acid" Organic Synthesis 1943, Collective Volume 2, page 302. Online version
- ↑ British Patent No. 775,912, publicated on the May 29, 1957, by Monsanto Chemical Company.
- ↑ Active Ingredients Used in Cosmetics: Safety Survey, Council of Europe. Committee of Experts on Cosmetic Products
- ↑ UK Food Standards Agency: "Current EU approved additives and their E Numbers". Retrieved 2011-10-27.
- ↑ US Food and Drug Administration: "Listing of Food Additives Status Part II". Retrieved 2011-10-27.
- ↑ Australia New Zealand Food Standards Code"Standard 1.2.4 - Labelling of ingredients". Retrieved 2011-10-27.
- ↑ http://www.seriouseats.com/2012/09/the-best-salt-and-vinegar-chips-tasting-brands-most-acidic.html
- ↑ Gold R.; Kappos L.; Arnold D.L.; et al. (September 20, 2012). "Placebo-Controlled Phase 3 Study of Oral BG-12 for Relapsing Multiple Sclerosis". N Engl J Med. 367 (12): 1098–1107. doi:10.1056/NEJMoa1114287. PMID 22992073.
- ↑ Balak DM, Fallah Arani S, Hajdarbegovic E, Hagemans CA, Bramer WM, Thio HB, Neumann HA (2016). "Efficacy, effectiveness, and safety of fumaric acid esters in the treatment of psoriasis: a systematic review of randomized and observational studies". Br. J. Dermatol. doi:10.1111/bjd.14500. PMID 26919824.
- ↑ Amenta R, Camarda L, Di Stefano V, Lentini F, Venza F (2000). "Traditional medicine as a source of new therapeutic agents against psoriasis". Fitoterapia. 71 Suppl 1: S13–20. doi:10.1016/s0367-326x(00)00172-6. PMID 10930708.
- ↑ Yot, Pascal G.; Vanduyfhuys L., Alvarez E.; Rodriguez J., Itié J.-P.; Fabry P., Guillou N.; Devic T., Beurroies I.; Llewellyn P.-L., Van Speyerbroeck V.; Serre C., Maurin G.; et al. (2016). "Mechanical energy storage performance of an aluminium fumarate metal-organic framework". Chem. Sci. 7 (1): 446–450. doi:10.1039/C5SC02794B.
- ↑ European Commission: "European Commission Report of the Scientific Committee on Animal Nutrition on the Safety of Fumaric Acid" (PDF). Retrieved 2014-03-07.
External links
| Citric acid cycle metabolic pathway | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxaloacetate | Malate | Fumarate | Succinate | Succinyl-CoA | ||||||||||||
| |
|
|
|
|
||||||||||||
| |
|
|
|
|||||||||||||
| Acetyl-CoA | NADH + H+ | NAD+ | H2O | FADH2 | FAD | CoA + ATP(GTP) | Pi + ADP(GDP) | |||||||||
| + | H2O | |
|
NADH + H+ + CO2 | ||||||||||||
| CoA | NAD+ | |||||||||||||||
| |
H2O | |
H2O | |
NAD(P)+ | NAD(P)H + H+ | |
CO2 | |
|||||||
| |
|
|
|
|||||||||||||
| Citrate | cis-Aconitate | Isocitrate | Oxalosuccinate | α-Ketoglutarate | ||||||||||||
| Urea cycle Metabolic Pathway | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||