Frustrated Lewis pair
In chemistry, a frustrated Lewis pair (FLP) is a compound or mixture containing a Lewis acid and a Lewis base that, because of steric hindrance, cannot combine to form a classical adduct.[1] Many kinds of FLP's have been devised, and many simple substrates exhibit activation.
The discovery that some FLPs split H2[2] triggered a rapid growth of research into FLP's. Because of their "unquenched" reactivity, such systems are reactive toward substrates that can undergo heterolysis. For example, many FLP's split hydrogen molecule. Thus, a mixture of tricyclohexylphosphine (PCy3) and tris(pentafluorophenyl)borane reacts with hydrogen to give the respective phosphonium and borate ions:
- PCy3 + B(C6F5)3 + H2 → [HPCy3]+[HB(C6F5)3]−
This reactivity suggests that FLP's are potentially useful for hydrogenation reactions.
Small Molecule Activation
Frustrated Lewis pairs can activate many small molecules, which can undergo heterolysis or are subject to polarization. The activation (i.e. reaction of) "small molecule" is an area of interest owing to the abundance of many small molecules. There is, for example, interest in use of N2 and O2 in the direct formation of C-N and C-O bonds. It also enables the utilization of environmentally damaging CO2 and CH4.[3]
Dihydrogen case
The activation H2 using FLPs was first reported in 2006. The hydrogen adduct of the original FLP, a phosphonium-borate salt, is prepared by combining a phenylene bridged phosphinoborane and dihydrogen. The salt, which is colorless, is stable in the presence of air and moisture. It releases molecular H2 when heated above 100 °C.
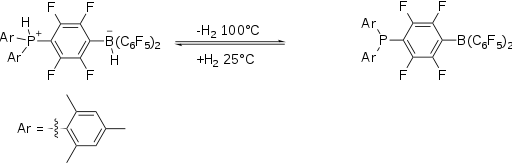
Along with H2 Some FLPs react with CO2. Specifically carbon dioxide fixation was demonstrated using P(tBu)3 which attacks the electronegative carbon and the Lewis acid B(C6F5)3 which accepts an electron pair from an oxygen atom:[4]
P(t-Bu)3 + B(C6F5)3 + CO2 → (t-Bu)3P+C(O)OB−(C6F5)3
The reaction is also reversible: the initially observed solid precipitate is stable up to 80 °C.[4]
Other small molecule substrates
FLPs are also reactive toward many unsaturated substrates. One example is ethylene:[5]
- PCy3 + B(C6F5)3 + C2H4 → Cy3P+CH2CH2B−(C6F5)3
For acid-base pairs to behave both nucleophilically and electrophilically at the same time offers a method for the ring-opening of cyclic ethers such as THF, 2,5-dihydrofuran, coumaran, and dioxane.[6]
Use in catalysis
Imine hydrogenation
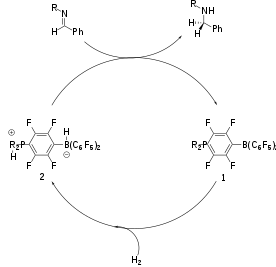
Reduction of imines and nitriles to give primary and secondary amines traditionally is effected by metal hydride reagents, e.g. lithium aluminium hydride and sodium cyanoborohydride. Hydrogenations of these unsaturated substrates can be effected by metal-catalyzed reactions. Metal-free catalytic hydrogenation was carried out using the phosphonium borate catalyst (R2PH)(C6F4)BH(C6F5)2 (R = 2,4,6-Me3C6H2) 1.[7]
During reduction of imines, proton at nitrogen facilitates the reaction. For this reason the basicity of the nitrogen centre determines the rate of reaction. More electron rich imines reduce at faster rates than electron poor imines. The protonated imine undergoes nucleophilic attack by the borohydride anion to form the amine. Dissociation of the amine is hindered by formation of the amine-borane adduct. This can be overcome using various methods: 1) Application of elevated temperatures 2) Using sterically bulky imine substituents 3) Protecting the imine with the B(C6F5)3 group, which also serves as a Lewis acid promoter.
Enantioselective imine hydrogenation
Chiral boronate Lewis acid derived from (1R)-(+)-camphor form a frustrated Lewis pair with tBu3P, which is isolable as a salt. This FLP catalyses the enantioselective hydrogenation of some aryl imines in high yield and good ee (up to 83%).
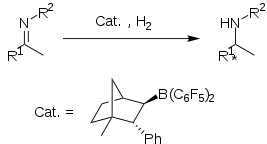
Although conceptually interesting, the protocol suffers from lack of generality. It was found that increasing steric bulk of the imine substituents lead to decreased yield and ee of the amine product. methoxy-substituted imines exhibit to superior yield and ee's.[8]
Alkyne Hydrogenation
Catalytic hydrogenation of internal alkynes to cis-alkenes is readily achieved using FLP-based catalysts.[9] In terms of mechanism, the alkyne material is first hydroborated. The resulting vinylborane-base FLP can then activate dihydrogen before a protodeborylation step releases the product, regenerating the catalyst.
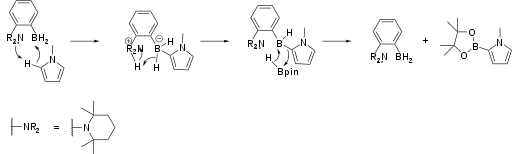
Borylation
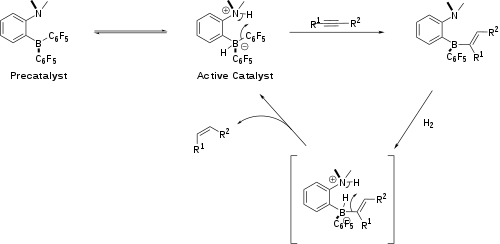
Amine-borane FLPs catalyse the borylation of electron-rich aromatic heterocycles (Scheme 1).[10] The reaction is driven by release of hydrogen via C-H activation by the FLP. Aromatic borylations of this type are often used in pharmaceutical development.
References
- ↑ Stephan, Douglas W. "Frustrated Lewis pairs": a concept for new reactivity and catalysis. Org. Biomol. Chem. 2008, 6, 1535-1539. doi:10.1039/b802575b
- ↑ Welch, Gregory C.; Juan, Ronan R. San; Masuda, Jason D.; Stephan, Douglas W. (2006-11-17). "Reversible, Metal-Free Hydrogen Activation". Science. 314 (5802): 1124–1126. doi:10.1126/science.1134230. ISSN 0036-8075.
- ↑ Milani, Barbara; Licini, Giulia; Clot, Eric; Albrecht, Martin (2016-09-20). "Small molecule activation". Dalton Transactions. 45 (37). doi:10.1039/C6DT90140A. ISSN 1477-9234.
- 1 2 Stephan, D. W.; Erker, G., "Frustrated Lewis Pair Chemistry: Development and Perspectives", Angew. Chem., Int. Ed. 2015, 54, 6400-6441. doi:10.1002/anie.201409800
- ↑ Stephan, D. W., ""Frustrated Lewis Pairs": A New Strategy to Small Molecule Activation and Hydrogenation Catalysis", Dalton Trans. 2009, 3129-3136. doi:10.1039/b819621d
- ↑ Tochertermam, W. Angew. Chem. Int. Ed., 1966, 5, 351-171
- ↑ Chase, Preston A.; Welch, Gregory C.; Jurca, Titel; Stephan, Douglas W. (2007-10-22). "Metal-Free Catalytic Hydrogenation". Angewandte Chemie International Edition. 46 (42): 8050–8053. doi:10.1002/anie.200702908. ISSN 1521-3773.
- ↑ Chen, Dianjun; Wang, Yutian; Klankermayer, Jürgen (2010-12-03). "Enantioselective Hydrogenation with Chiral Frustrated Lewis Pairs". Angewandte Chemie International Edition. 49 (49): 9475–9478. doi:10.1002/anie.201004525. ISSN 1521-3773.
- ↑ Chernichenko, Konstantin; Madarász, Ádám; Pápai, Imre; Nieger, Martin; Leskelä, Markku; Repo, Timo. "A frustrated-Lewis-pair approach to catalytic reduction of alkynes to cis-alkenes". Nature Chemistry. 5 (8): 718–723. doi:10.1038/nchem.1693.
- ↑ Légaré, Marc A.; Courtmanche, Marc A.; Rochette, Étienne; Fontaine, Frédéric G. (2015-07-30). "Metal-free catalytic C-H bond activation and borylation of heteroarenes" (PDF). doi:10.1126/science.aab3591. ISSN 0036-8075.