Monotreme
| Monotremes[1] Temporal range: Late Triassic[2]–Holocene, 210–0 Ma | |
|---|---|
 | |
| A short-beaked echidna, a platypus, a Steropodon reconstruction and a western long-beaked echidna | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Clade: | Synapsida |
| Class: | Mammalia |
| Infraclass: | Australosphenida |
| Order: | Monotremata C.L. Bonaparte, 1837 |
| Subgroups | |
Monotremes are mammals that lay eggs (Prototheria) instead of giving birth to live young like marsupials (Metatheria) and placental mammals (Eutheria). The only surviving examples of monotremes are all indigenous to Australia and New Guinea, although there is evidence that they were once more widespread. The existing monotreme species are the platypus and four species of echidnas. There is currently some debate regarding monotreme taxonomy.
The word monotreme comes from the Greek μονός, monos ("single") and τρῆμα, trema ("hole"), referring to the cloaca.
General characteristics
Like other mammals, monotremes are warm-blooded with a high metabolic rate (though not as high as other mammals; see below); have hair on their bodies; produce milk through mammary glands to feed their young; have a single bone in their lower jaw; and have three middle-ear bones.
In common with reptiles and marsupials, monotremes lack the connective structure (corpus callosum) which in placental mammals is the primary communication route between the right and left brain hemispheres.[3] The anterior commissure does provide an alternate communication route between the two hemispheres, though, and in monotremes and marsupials it carries all the commissural fibers arising from the neocortex, whereas in placental mammals the anterior commissure carries only some of these fibers.[4]

Extant monotremes lack teeth as adults. Fossil forms and modern platypus young have a "tribosphenic" form of molars (with the occlusal surface formed by three cusps arranged in a triangle), which is one of the hallmarks of extant mammals. Some recent work suggests that monotremes acquired this form of molar independently of placental mammals and marsupials,[5] although this is not well established.[6] Toothloss in modern monotremes might be related to their development of electrolocation.[7]
Monotreme jaws are constructed somewhat differently from those of other mammals, and the jaw opening muscle is different. As in all true mammals, the tiny bones that conduct sound to the inner ear are fully incorporated into the skull, rather than lying in the jaw as in cynodonts and other premammalian synapsids; this feature, too, is now claimed to have evolved independently in monotremes and therians,[8] although, as with the analogous evolution of the tribosphenic molar, this is disputed.[9][10] Nonetheless, findings on the extinct species Teinolophos confirm that suspended ear bones evolved independently among monotremes and therians.[11] The external opening of the ear still lies at the base of the jaw.
The sequencing of the platypus genome has also provided insight into the evolution of a number of monotreme traits, such as venom and electroreception, as well as showing some new unique features, such as the fact that monotremes possess 10 sex chromosomes and that their X chromosome resembles the sex chromosome of birds,[12] suggesting that the two sex chromosomes of marsupial and placental mammals evolved more recently than the split from the monotreme lineage.[13] This feature, along with some other genetic similarities with birds, such as shared genes related to egg-laying, is thought to provide some insight into the most recent common ancestor of the synapsid lineage leading to mammals and the sauropsid lineage leading to birds and modern reptiles, which are believed to have split about 315 million years ago during the Carboniferous.[14][15] The presence of vitellogenin genes (a protein necessary for egg shell formation) is shared with birds, suggesting that when the common ancestor of mammals from ~225 million years ago split into monotremes, marsupials, and placental mammals, egg laying was retained in monotremes and lost in all other mammals. DNA suggests that while this trait is shared and is synapomorphic with birds, platypuses are still mammals and they evolved lactation with other mammals.[16] L-ascorbic acid is synthesized only in the kidneys.[17]
The monotremes also have extra bones in the shoulder girdle, including an interclavicle and coracoid, which are not found in other mammals. Monotremes retain a reptile-like gait, with legs on the sides of, rather than underneath, their bodies. The monotreme leg bears a spur in the ankle region; the spur is not functional in echidnas, but contains a powerful venom in the male platypus. This venom is derived from b-defensins, proteins that are present in mammals that create holes in viral and bacterial pathogens. Some reptile venom is also composed of different types of b-defensins, another trait shared with reptiles.[18] It is thought to be an ancient mammalian characteristic, as many non-monotreme archaic mammal groups also possess venomous spurs.[19]
Reproductive system
The key anatomical difference between monotremes and other mammals is what lends the animals their name; monotreme means “single opening” in Greek, derived from the fact that their urinary, defecatory, and reproductive systems all open into a single duct, called the cloaca; and this anatomical structure is very similar to the one found in reptiles. Monotremes and marsupials have a single cloaca (though marsupial reproductive systems also have a separate genital tract), while most placental mammal females have separate openings for reproduction, urination, and defecation, being the vagina, the urethra, and the anus, respectively. The penis only carries semen, urine is excreted through the cloaca.[20]
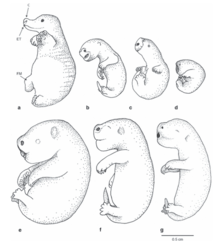
Monotremes lay eggs. However, the egg is retained for some time within the mother, which actively provides the egg with nutrients, and generally hatches soon after birth, within the span of 10 days (as opposed to eggs of sauropsids, which generally take much longer to incubate).[21][22] Newborn monotremes are larval and fetus-like, much like those of marsupials (and, indeed, perhaps all non-placental mammals[23]), and like them also bear relatively well developed forelimbs to crawl around. In fact, given that monotremes lack nipples, puggles crawl about more frequently than marsupial joeys in search of milk, raising questions about the supposed development restrictions on marsupial forelimbs.[24]
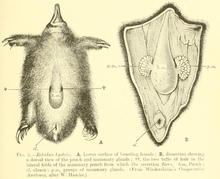
Monotremes, like all mammals, lactate, but have no defined nipples, excreting the milk from their mammary glands via openings in their skin. All species are long-lived, with low rates of reproduction and relatively prolonged parental care of infants.
Another noteworthy trait of monotremes involves their developmental characteristics, such as the zygotic development of platypuses: Most mammal zygotes go through holoblastic cleavage, meaning that following fertilization, the ovum is split due to cell divisions into multiple, divisible daughter cells. The zygotes of monotremes, however, undergo meroblastic division (as in birds and reptiles), which causes the ovum to split but not completely. This means the cells at the edge of the yolk are cytoplasmically continuous with the egg's cytoplasm, thereby allowing the yolk, which contains the embryo, to exchange waste and nutrients with the cytoplasm.[25]
Physiology

Monotremes' metabolic rate is remarkably low by mammalian standards. The platypus has an average body temperature of about 31 °C (88 °F) rather than the averages of 35 °C (95 °F) for marsupials and 37 °C (99 °F) for placental mammals.[26][27] Research suggests this has been a gradual adaptation to harsh environmental conditions on the part of the small number of surviving monotreme species rather than a historical characteristic of monotremes.[28][29]
Monotremes may have less developed thermoregulation than other mammals, but recent research shows that they maintain a constant body temperature in a wide variety of circumstances without difficulty (for example, the platypus while living in an icy mountain stream). Early researchers were misled by two factors: firstly, monotremes maintain a lower average temperature than most mammals; secondly, the short-beaked echidna (which is much easier to study than the reclusive platypus) maintains normal temperature only when it is active; during cold weather, it conserves energy by "switching off" its temperature regulation. Additional perspective came when reduced thermal regulation was observed in the hyraxes, which are placental mammals.
The echidna was originally thought to not enter rapid eye movement sleep.[30] However, a more recent study showed that REM sleep accounted for about 15% of the total sleep time observed on subjects at an environmental temperature of 25 °C (77 °F). Surveying a range of environmental temperatures, the study observed very little REM at reduced temperatures of 15 °C (59 °F) and 20 °C (68 °F), and also a substantial reduction at the elevated temperature of 28 °C (82 °F).[31]
Monotreme milk contains a highly expressed antibacterial protein not found in other mammals, perhaps to compensate for the less sterile manner of milk intake associated with the absence of nipples.[32]
Taxonomy
Monotremes are conventionally treated as comprising a single order Monotremata, though a recent classification[33] proposes to divide them into the orders Platypoda (the platypus along with its fossil relatives) and Tachyglossa (the echidnas, or spiny anteaters). The entire grouping is also traditionally placed into a subclass Prototheria, which was extended to include several fossil orders, but these are no longer seen as constituting a group allied to monotreme ancestry. A controversial hypothesis now relates the monotremes to a different assemblage of fossil mammals in a clade termed Australosphenida.[5][34]
The traditional "theria hypothesis" states that the divergence of the monotreme lineage from the Metatheria (marsupial) and Eutheria (placental mammal) lineages happened prior to the divergence between marsupials and placental mammals, and this explains why monotremes retain a number of primitive traits presumed to have been present in the synapsid ancestors of later mammals, such as egg-laying.[35][36][37] Most morphological evidence supports the theria hypothesis, but one possible exception is a similar pattern of tooth replacement seen in monotremes and marsupials, which originally provided the basis for the competing "marsupionata hypothesis" in which the divergence between monotremes and marsupials happened later than the divergence between these lineages and the placental mammals. An analysis by Van Rheede in 2005 concluded that the genetic evidence favors the theria hypothesis,[38] and this hypothesis continues to be the more widely accepted one.[39]
The time when the monotreme line diverged from other mammalian lines is uncertain, but one survey of genetic studies gives an estimate of about 220 million years ago.[40] Fossils of a jaw fragment 110 million years old were found at Lightning Ridge, New South Wales. These fragments, from the species Steropodon galmani, are the oldest known fossils of monotremes. Fossils from the genera Teinolophos, and Obdurodon have also been discovered. In 1991, a fossil tooth of a 61-million-year-old platypus was found in southern Argentina (since named Monotrematum, though it is now considered to be an Obdurodon species). (See fossil monotremes below.) Molecular clock and fossil dating give a wide range of dates for the split between echidnas and platypuses, with one survey putting the split at 19–48 million years ago,[41] but another putting it at 17–89 million years ago.[42] All these dates are more recent than the oldest known platypus fossils, suggesting that both the short-beaked and long-beaked echidna species are derived from a platypus-like ancestor. The earliest echidna found to date is about 13 million years.[43]
The precise relationships between extinct groups of mammals and modern groups, such as monotremes, are somewhat uncertain, but cladistic analyses usually put the last common ancestor (LCA) of placentals and monotremes close to the LCA of placentals and multituberculates, with a number of analyses giving a more recent LCA for placentals and monotremes, but some also suggesting the LCA of placentals and multituberculates was more recent.[44][45]

- ORDER MONOTREMATA
- Suborder Platypoda
- Family Ornithorhynchidae: platypus
- Genus Ornithorhynchus
- Platypus, O. anatinus
- Genus Ornithorhynchus
- Family Ornithorhynchidae: platypus
- Suborder Tachyglossa
- Family Tachyglossidae: echidnas
- Genus Tachyglossus
- Short-beaked echidna, T. aculeatus
- T. a. aculeatus
- T. a. acanthion
- T. a. lawesii
- T. a. multiaculeatus
- T. a. setosus
- Short-beaked echidna, T. aculeatus
- Genus Zaglossus
- Sir David's long-beaked echidna, Z. attenboroughi
- Eastern long-beaked echidna, Z. bartoni
- Z. b. bartoni
- Z. b. clunius
- Z. b. diamondi
- Z. b. smeenki
- Western long-beaked echidna, Z. bruijni
- Genus Tachyglossus
- Family Tachyglossidae: echidnas
- Suborder Platypoda
Fossil monotremes
The fossil record of monotremes is relatively sparse. The first Mesozoic monotreme to be discovered was Steropodon galmani from Lightning Ridge, New South Wales.[46] Although biochemical and anatomical evidence suggests that the monotremes diverged from the mammalian lineage before the marsupials and placental mammals arose, only a handful of monotreme fossils are known from before the Miocene epoch. The known Mesozoic monotremes are Steropodon and Teinolophos, all from Australian deposits in the Cretaceous, suggesting monotremes had already diversified by that time.[47] A platypus tooth has been found in the Palaeocene of Argentina, so Michael Benton suggests in Vertebrate Palaeontology monotremes arose in Australia in the Late Jurassic or Early Cretaceous, and some subsequently migrated across Antarctica to reach South America, both of which were still united with Australia at that time.[48] However, a number of genetic studies suggest a much earlier origin in the Triassic.[49]
Fossil species


Excepting Ornithorhynchus anatinus, all the animals listed in this section are known only from fossils.
- Genus Kryoryctes
- Species Kryoryctes cadburyi
- Genus Kryoryctes
- Family Steropodontidae – paraphyletic assemblage
- Genus Steropodon
- Species Steropodon galmani
- Genus Teinolophos
- Species Teinolophos trusleri – 123 million years old, oldest monotreme specimen
- Genus Steropodon
- Family Ornithorhynchidae
- Genus Ornithorhynchus – oldest Ornithorhynchus specimen 9 million years old
- Species Ornithorhynchus anatinus (platypus) – oldest specimen 10,000 years old
- Genus Obdurodon – includes a number of Miocene (5–24 million years ago) platypuses
- Species Obdurodon dicksoni (Riversleigh platypus)
- Species Obdurodon insignis
- Species Obdurodon tharalkooschild – middle and upper Miocene (15–5 mya)
- Species Monotrematum sudamericanum – 61 million years old, originally placed in separate genus, now thought an Obdurodon
- Genus Ornithorhynchus – oldest Ornithorhynchus specimen 9 million years old
- Family Tachyglossidae
- Genus Zaglossus Upper Pleistocene (0.1–1.8 million years ago)
- Species Zaglossus hacketti
- Species Zaglossus robustus
- Genus Megalibgwilia
- Megalibgwilia ramsayi Late Pleistocene
- Megalibgwilia robusta Miocene
- Genus Zaglossus Upper Pleistocene (0.1–1.8 million years ago)
References
- ↑ Groves, C.P. (2005). Wilson, D.E.; Reeder, D.M., eds. Mammal Species of the World: A Taxonomic and Geographic Reference (3rd ed.). Baltimore: Johns Hopkins University Press. pp. 1–2. OCLC 62265494. ISBN 0-801-88221-4.
- ↑ Hugall, A.F.; et al. (2007). "Calibration choice, rate smoothing, and the pattern of tetrapod diversification according to the long nuclear gene RAG-1". Syst Biol. 56 (4): 543–63. doi:10.1080/10635150701477825.
- ↑ "Animal Bytes – Order Monotremata".
- ↑ Butler, Ann B., and William Hodos (2005). Comparative Vertebrate Neuroanatomy: Evolution and Adaptation, p. 361
- 1 2 Luo, Z.-X.; Cifelli, R.L.; Kielan-Jaworowska, Z. (2001). "Dual origin of tribosphenic mammals". Nature. 409 (6816): 53–57. doi:10.1038/35051023. PMID 11343108.
- ↑ "Mammalian evolution: Relationships to chew over.". Nature. 409: 28–31. 2001-01-04. doi:10.1038/35051199.
- ↑ Masakazu Asahara; Masahiro Koizumi; Thomas E. Macrini; Suzanne J. Hand; Michael Archer (2016). "Comparative cranial morphology in living and extinct platypuses: Feeding behavior, electroreception, and loss of teeth". Science Advances. 2 (10): e1601329. doi:10.1126/sciadv.1601329.
- ↑ Rich, T. H.; Hopson, J. A.; Musser, A. M.; Flannery, T. F.; Vickers-Rich, P. (2005). "Independent origins of middle ear bones in monotremes and therians". Science. 307 (5711): 910–914. doi:10.1126/science.1105717. PMID 15705848.
- ↑ "Comment on "Independent Origins of Middle Ear Bones in Monotremes and Therians" (I)". Science Magazine. Retrieved 2007-10-21.
- ↑ "Comment on "Independent Origins of Middle Ear Bones in Monotremes and Therians" (II)". Science Magazine. Retrieved 2007-10-21.
- ↑ Thomas H. Rich, James A. Hopson, Pamela G. Gill, Peter Trusler, Sally Rogers-Davidson, Steve Morton, Richard L. Cifelli, David Pickering, Lesley Kool, Karen Siu, Flame A. Burgmann, Tim Senden, Alistair R. Evans, Barbara E. Wagstaff, Doris Seegets-Villiers, Ian J. Corfe, Timothy F. Flannery, Ken Walker, Anne M. Musser, Michael Archer, Rebecca Pian and Patricia Vickers-Rich (2016). "The mandible and dentition of the Early Cretaceous monotreme Teinolophos trusleri". Alcheringa: An Australasian Journal of Palaeontology. in press. doi:10.1080/03115518.2016.1180034.
- ↑ "Platypus Genome Explains Animal's Peculiar Features; Holds Clues To Evolution Of Mammals". Sciencedaily.com. 2008-05-07. Retrieved 2011-06-09.
- ↑ Veyrunes et al. "Bird-like sex chromosomes of platypus imply recent origin of mammal sex chromosomes", Genome Res. 2008 June; 18(6): 965–973
- ↑ "Interpreting Shared Characteristics: The Platypus Genome | Learn Science at Scitable". Nature.com. Retrieved 2011-06-09.
- ↑ "Genome analysis of the platypus reveals unique signatures of evolution". Nature. 453 (7192): 175–183. doi:10.1038/nature06936. PMC 2803040
 . PMID 18464734. Retrieved 2011-06-09.
. PMID 18464734. Retrieved 2011-06-09. - ↑ 5. Brawand, D, W Wahli, and H Kaessmann. "Loss of Egg Yolk Genes in Mammals and the Origin of Lactation and Placentation." Plos Biology. 6.3 (2008). Print.
- ↑ Ascorbic acid biosynthesis in the mammalian kidney
- ↑ Myers, Pz. "Interpreting Shared Characteristics: The Platypus Genome." Nature.com. Nature Publishing Group, n.d. (2008) Web. 28 Oct. 2014.
- ↑ Jørn H. Hurum, Zhe-Xi Luo, and Zofia Kielan-Jaworowska, Were mammals originally venomous?, Acta Palaeontologica Polonica 51 (1), 2006: 1-11
- ↑ Human Sperm Competition: Copulation, masturbation and infidelity
- ↑ Cromer, Erica (14 April 2004). "Monotreme Reproductive Biology and Behavior". Iowa State University. Retrieved 18 June 2009.
- ↑ "Short-beaked echidna (Tachyglossus aculeatus)". Arkive.org.
- ↑ Michael L. Power,Jay Schulkin. The Evolution Of The Human Placenta. pp. 68–.
- ↑ Nanette Yvette Schneider, The development of the olfactory organs in newly hatched monotremes and neonate marsupials, J Anat. 2011 Aug; 219(2): 229–242. Published online 2011 May 17. doi: 10.1111/j.1469-7580.2011.01393.x
- ↑ 3. Myers, Pz. "Interpreting Shared Characteristics: The Platypus Genome." Nature.com. Nature Publishing Group, n.d. (2008) Web. 28 Oct. 2014.
- ↑ "Thermal Biology of the Platypus". Davidson College. 1999. Archived from the original on March 6, 2012. Retrieved 2006-09-14.
- ↑ "Control Systems Part 2" (PDF). Retrieved 6 July 2016.
- ↑ J.M. Watson and J.A.M. Graves (1988). "Monotreme Cell-Cycles and the Evolution of Homeothermy". Australian Journal of Zoology. CSIRO. 36 (5): 573–584. doi:10.1071/ZO9880573.
- ↑ T.J. Dawson, T.R. Grant and D. Fanning (1979). "Standard Metabolism of Monotremes and the Evolution of Homeothermy". Australian Journal of Zoology. CSIRO. 27 (4): 511–515. doi:10.1071/ZO9790511.
- ↑ Siegel, J. M.; Manger, P. R.; Nienhuis, R.; Fahringer, H. M.; Pettigrew, J. D. (1998). "Monotremes and the evolution of rapid eye movement sleep". Philosophical Transactions of the Royal Society B: Biological Sciences. 353 (1372): 1147–1157. doi:10.1098/rstb.1998.0272. PMC 1692309
 . PMID 9720111.
. PMID 9720111. - ↑ Nicol, S. C.; Andersen, N. A.; Phillips, N. H.; Berger, R. J. (2000). "The echidna manifests typical characteristics of rapid eye movement sleep". Neuroscience Letters. 283 (1): 49–52. doi:10.1016/S0304-3940(00)00922-8. PMID 10729631.
- ↑ Bisana, S.; Kumar, S.; Rismiller, P.; Nicol, S. C.; Lefèvre, C.; Nicholas, K. R.; Sharp, J. A. (2013-01-09). "Identification and Functional Characterization of a Novel Monotreme- Specific Antibacterial Protein Expressed during Lactation". PLoS ONE. 8 (1): e53686. doi:10.1371/journal.pone.0053686. PMC 3541144
 . PMID 23326486.
. PMID 23326486. - ↑ McKenna, Malcolm C., and Susan K. Bell (1997). Classification of Mammals Above the Species Level. New York: Columbia University Press. 631 pp. ISBN 0-231-11013-8
- ↑ Luo, Z.-X.; Cifelli, R.L.; Kielan-Jaworowska, Z. (2002). "In quest for a phylogeny of Mesozoic mammals". Acta Palaeontologica Polonica. 47: 1–78.
- ↑ Terry A. Vaughan, James M. Ryan, Nicholas J. Czaplewski: Mammalogy, Fifth Edition (2010), p. 80
- ↑ "Introduction to the Monotremata". Ucmp.berkeley.edu. Retrieved 2011-06-09.
- ↑ http://www.webpages.uidaho.edu/~jacks/Lecture3.pdf
- ↑ Van Rheede, Teun (2005). "The Platypus Is in Its Place: Nuclear Genes and Indels Confirm the Sister Group Relation of Monotremes and Therians". Molecular Biology and Evolution. 23 (3): 587–597. doi:10.1093/molbev/msj064. PMID 16291999.
- ↑ "Monotremes". Tolweb.org. doi:10.1073/pnas.94.4.1276. Retrieved 2011-06-09.
- ↑ Madsen, Ole. "Mammals". In Hedges, S. Blair. The Timetree of Life.
- ↑ Phillips, MJ; Bennett, TH; Lee, MS. (2009). "Molecules, morphology, and ecology indicate a recent, amphibious ancestry for echidnas". PNAS. 106: 17089–17094. doi:10.1073/pnas.0904649106. PMC 2761324
 . PMID 19805098.
. PMID 19805098. - ↑ http://timetree.org/pdf/Springer2009Chap69.pdf
- ↑ Echidna and platypus share common ancestor: research
- ↑ Benton, Michael J. Vertebrate Palaeontology (2004), p. 300
- ↑ Carrano, Matthew T., and Richard W. Blob, Timothy J. Gaudin, and John R. Wible (2006). Amniote Paleobiology: Perspectives on the Evolution of Mammals, Birds, and Reptiles, p. 358.
- ↑ Ashwell, K, ed. (2013). Neurobiology of Monotremes. Melbourne: CSIRO Publishing. ISBN 9780643103115.
- ↑ "Fossil Record of the Monotremata". Ucmp.berkeley.edu. Retrieved 2011-06-09.
- ↑ Benton, M.J. (1997). Vertebrate Palaeontology. London: Chapman & Hall. pp. 303–304. ISBN 0-412-73810-4.
- ↑ http://timetree.org/pdf/Madsen2009Chap68.pdf
Bibliography
- Ronald M. Nowak (1999), Walker’s Mammals of the World (6 ed.), Baltimore: Johns Hopkins University Press, ISBN 0-8018-5789-9, LCCN 98023686
External links
| The Wikibook Dichotomous Key has a page on the topic of: Monotremata |


