Fibromyalgia
| Fibromyalgia | |
|---|---|
| fibromyalgia syndrome (FMS) | |
|
The location of the nine paired tender points that constitute the 1990 American College of Rheumatology criteria for fibromyalgia. | |
| Classification and external resources | |
| Pronunciation | /ˌfaɪbroʊmaɪˈældʒə/[1] |
| Specialty | Rheumatology |
| ICD-10 | M79.7 – Fibromyalgia |
| ICD-9-CM | 729.1 – Myalgia and myositis, unspecified |
| MedlinePlus | 000427 |
| eMedicine | med/790 med/2934 ped/777 pmr/47 |
| Patient UK | Fibromyalgia |
| MeSH | D005356 |
Fibromyalgia (FM) is a medical condition characterised by chronic widespread pain and a heightened pain response to pressure.[2] Other symptoms include feeling tired to a degree that normal activities are affected, sleep problems, and troubles with memory.[3] Some people also report restless legs syndrome, bowel or bladder problems, numbness and tingling, and sensitivity to noise, lights or temperature.[4] Fibromyalgia is frequently associated with depression, anxiety, and posttraumatic stress disorder. Other types of chronic pain are also frequently present.[3]
The cause of fibromyalgia is unknown but believed to involve a combination of genetic and environmental factors with half the risk attributed to each.[3][4] The condition runs in families and many genes are believed to be involved.[5] Environmental factors may include psychological stress, trauma, and certain infections.[3] The pain appears to result from processes in the central nervous system and the condition is referred to as a "central sensitization syndrome".[2][3] Fibromyalgia is recognized as a disorder by the US National Institutes of Health and the American College of Rheumatology.[4][6] There is no specific diagnostic test.[4] Diagnosis involves first ruling out other potential causes and verifying that a set number of symptoms are present.[3][4]
The treatment of fibromyalgia can be difficult. Recommendations often include getting enough sleep, exercising regularly, and eating a healthy diet.[4] Cognitive behavioral therapy may also be helpful.[3] The medications duloxetine, milnacipran, or pregabalin may be used.[4] Use of opioid pain medication is controversial with some stating their use is poorly supported by evidence[4][7] and others saying that weak opioids may be reasonable if other medications are not effective.[8] Dietary supplements also lack evidence to support their use. While fibromyalgia can last a long time, it does not result in death or tissue damage.[4]
Fibromyalgia is estimated to affect 2–8% of the population. Females are affected about twice as often as males. Rates appear similar in different areas of the world and among different cultures. Fibromyalgia was first defined in 1990 with updated criteria in 2011.[3] There is controversy about the classification, diagnosis, and treatment of fibromyalgia.[9][10] While some feel the diagnosis of fibromyalgia may negatively affect a person, other research finds it to be beneficial.[3] The term "fibromyalgia" is from New Latin, fibro-, meaning "fibrous tissues", Greek μυώ myo-, "muscle", and Greek άλγος algos, "pain"; thus the term literally means "muscle and fibrous connective tissue pain".[11]
Classification
Fibromyalgia is classed as a disorder of pain processing due to abnormalities in how pain signals are processed in the central nervous system.[12] The American College of Rheumatology classify fibromyalgia as being a functional somatic syndrome.[9] The expert committee of the European League Against Rheumatism classify fibromyalgia as a neurobiological disorder and as a result exclusively give pharmacotherapy their highest level of support.[9] The International Classification of Diseases (ICD-10) lists fibromyalgia as a diagnosable disease under "Diseases of the musculoskeletal system and connective tissue," under the code M79-7, and states that fibromyalgia syndrome should be classified as a functional somatic syndrome rather than a mental disorder. Although mental disorders and some physical disorders commonly are co-morbid with fibromyalgia – especially anxiety, depression, irritable bowel syndrome, and chronic fatigue syndrome – the ICD states that these should be diagnosed separately.[9]
Differences in psychological and autonomic nervous system profiles among affected individuals may indicate the existence of fibromyalgia subtypes. A 2007 review divides individuals with fibromyalgia into four groups as well as "mixed types":[13]
- "extreme sensitivity to pain but no associated psychiatric conditions" (may respond to medications that block the 5-HT3 receptor)
- "fibromyalgia and comorbid, pain-related depression" (may respond to antidepressants)
- "depression with concomitant fibromyalgia syndrome" (may respond to antidepressants)
- "fibromyalgia due to somatization" (may respond to psychotherapy)
Signs and symptoms
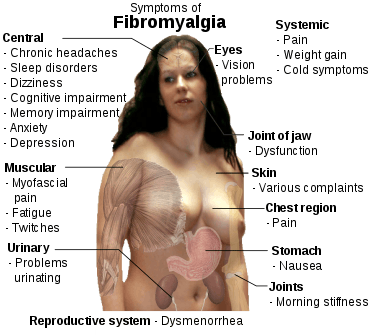
The defining symptoms of fibromyalgia are chronic widespread pain, fatigue, sleep disturbance, and heightened pain in response to tactile pressure (allodynia).[14] Other symptoms may include tingling of the skin (paresthesias),[14] prolonged muscle spasms, weakness in the limbs, nerve pain, muscle twitching, palpitations,[15] and functional bowel disturbances.[16][17]
Many people experience cognitive dysfunction[14][18] (known as "fibrofog"), which may be characterized by impaired concentration,[19] problems with short[19][20] and long-term memory, short-term memory consolidation,[20] impaired speed of performance,[19][20] inability to multi-task, cognitive overload,[19][20] and diminished attention span. Fibromyalgia is often associated with anxiety and depressive symptoms.[20]
Other symptoms often attributed to fibromyalgia that may be due to a comorbid disorder include myofascial pain syndrome, also referred to as chronic myofascial pain, diffuse non-dermatomal paresthesias, functional bowel disturbances and irritable bowel syndrome, genitourinary symptoms and interstitial cystitis, dermatological disorders, headaches, myoclonic twitches, and symptomatic hypoglycemia. Although fibromyalgia is classified based on the presence of chronic widespread pain, pain may also be localized in areas such as the shoulders, neck, low back, hips, or other areas. Many sufferers also experience varying degrees of myofascial pain and have high rates of comorbid temporomandibular joint dysfunction. 20–30% of people with rheumatoid arthritis and systemic lupus erythematosus may also have fibromyalgia.[21]
Cause
The cause of fibromyalgia is unknown. However, several hypotheses have been developed including "central sensitization".[14] This theory proposes that people with fibromyalgia have a lower threshold for pain because of increased reactivity of pain-sensitive nerve cells in the spinal cord or brain.[2] Neuropathic pain and major depressive disorder often co-occur with fibromyalgia – the reason for this comorbidity appears to be due to shared genetic abnormalities, which leads to impairments in monoaminergic, glutamatergic, neurotrophic, opioid and proinflammatory cytokine signaling. In these vulnerable individuals, psychological stress or illness can cause abnormalities in inflammatory and stress pathways which regulate mood and pain. Eventually, a sensitization and kindling effect occur in certain neurons leading to the establishment of fibromyalgia and sometimes a mood disorder.[22] The evidence suggests that the pain in fibromyalgia results primarily from pain processing pathways functioning abnormally. In simple terms, it can be described as the volume of the neurons being set too high and this hyper-excitability of pain processing pathways and under-activity of inhibitory pain pathways in the brain results in the affected individual experiencing pain. Some neurochemical abnormalities that occur in fibromyalgia also regulate mood, sleep, and energy, thus explaining why mood, sleep, and fatigue problems are commonly co-morbid with fibromyalgia.[12]
Genetics
A mode of inheritance is currently unknown, but it is most probably polygenic.[5] Research has also demonstrated that fibromyalgia is potentially associated with polymorphisms of genes in the serotoninergic,[23] dopaminergic[24] and catecholaminergic systems.[25] However, these polymorphisms are not specific for fibromyalgia and are associated with a variety of allied disorders (e.g. chronic fatigue syndrome,[26] irritable bowel syndrome[27]) and with depression.[28] Individuals with the 5-HT2A receptor 102T/C polymorphism have been found to be at increased risk of developing fibromyalgia.[29]
Lifestyle
Stress may be an important precipitating factor in the development of fibromyalgia.[30] Fibromyalgia is frequently comorbid with stress-related disorders such as chronic fatigue syndrome, posttraumatic stress disorder, irritable bowel syndrome and depression.[31] A systematic review found significant association between fibromyalgia and physical and sexual abuse in both childhood and adulthood, although the quality of studies was poor.[32] Poor lifestyles including being a smoker, obesity and lack of physical activity may increase the risk of an individual developing fibromyalgia.[33]
Two studies that employed single-voxel magnetic resonance spectroscopy (1H-MRS) reported metabolic abnormalities within the hippocampal complex in people with fibromyalgia. As the hippocampus plays crucial roles in maintenance of cognitive functions, sleep regulation, and pain perception, it was suggested that metabolic dysfunction of the hippocampus may be implicated in the appearance of these symptoms.[34][35]
Some authors have proposed that, because exposure to stressful conditions can alter the function of the hypothalamic-pituitary-adrenal (HPA) axis, the development of fibromyalgia may stem from stress-induced disruption of the HPA axis.[36]
Sleep disturbances
In 1975, Moldofsky and colleagues reported the presence of anomalous alpha wave activity (typically associated with arousal states) measured by electroencephalogram (EEG) during non-rapid eye movement sleep of "fibrositis syndrome".[17] By disrupting stage IV sleep consistently in young, healthy subjects, the researchers reproduced a significant increase in muscle tenderness similar to that experienced in "neurasthenic musculoskeletal pain syndrome" but which resolved when the subjects were able to resume their normal sleep patterns.[37]
Psychological factors
There is strong evidence that major depression is associated with fibromyalgia (1999),[38] although the nature of the association is debated.[39] A comprehensive review into the relationship between fibromyalgia and major depressive disorder (MDD) found substantial similarities in neuroendocrine abnormalities, psychological characteristics, physical symptoms and treatments between fibromyalgia and MDD, but currently available findings do not support the assumption that MDD and fibromyalgia refer to the same underlying construct or can be seen as subsidiaries of one disease concept.[40] Indeed, the sensation of pain has at least two dimensions: a sensory dimension which processes the magnitude and location of the pain, and an affective-motivational dimension which processes the unpleasantness. Accordingly, a study that employed functional magnetic resonance imaging to evaluate brain responses to experimental pain among people with fibromyalgia found that depressive symptoms were associated with the magnitude of clinically induced pain response specifically in areas of the brain that participate in affective pain processing, but not in areas involved in sensory processing which indicates that the amplification of the sensory dimension of pain in fibromyalgia occurs independently of mood or emotional processes.[41] Fibromyalgia has also been linked with bipolar disorder, particularly the hypomania component.[42]
Non-celiac gluten sensitivity
Non-celiac gluten sensitivity (NCGS) may be an underlying cause of fibromyalgia symptoms but further research is needed.[43][44]
Pathophysiology
The brains of people with fibromyalgia show functional and structural differences from those of people without fibromyalgia, but it is unclear whether the brain anomalies cause fibromyalgia symptoms, or are the product of an unknown underlying common cause. Some research suggests that these brain anomalies may be the result of childhood stress, or prolonged or severe stress.[31]
Dopamine dysfunction
The "dopamine hypothesis of fibromyalgia" proposes that the central abnormality responsible for symptoms associated with fibromyalgia is a disruption of normal dopamine-related neurotransmission.[45] Insufficient dopamine in a part of the body is termed hypodopaminergia. Dopamine is a catecholamine neurotransmitter with roles in pain perception and natural analgesia. There is also strong evidence for a role of dopamine in restless leg syndrome,[46] which is a condition found frequently in people with fibromyalgia.[47] Some people with fibromyalgia responded to pramipexole, a dopamine agonist that selectively stimulates dopamine D2/D3 receptors and is used to treat both Parkinson's disease and restless leg syndrome.[48]
Decreased CSF levels of dopamine were reported in 1992, which may explain the efficacy of some dopaminergic agents in fibromyalgia.[49]
Serotonin metabolism
Serotonin is a neurotransmitter involved in pathways that project to the dorsal horns and inhibit pain perception. In 1975, researchers hypothesized that serotonin could be involved in the pathophysiology of fibromyalgia-associated symptoms.[17] In 1992, decreased serotonin metabolites in people's blood samples[50] and cerebrospinal fluid were reported.[51][52] However, selective serotonin reuptake inhibitors (SSRIs) have met with limited success in alleviating the symptoms of the disorder, while drugs with activity as mixed serotonin-norepinephrine reuptake inhibitors (SNRIs) have been more successful.[53] However, the relevance of dysregulated serotonin metabolism to pathophysiology is a matter of debate.[54] Complicating the analysis, one of the more effective types of medication for the treatment of the disorder (i.e. serotonin 5-HT3 antagonists) actually blocks some effects of serotonin.[55]
Poly-modal sensitivity
Results from studies examining responses to experimental stimulation suggest that people with fibromyalgia may have heightened sensitivity of the nociceptive system, which senses pressure, heat, cold, electrical and chemical stimulation.[56] Experiments examining pain regulatory systems have shown that people with fibromyalgia display an exaggerated wind-up in response to repetitive stimulation[57] and an absence of exercise-induced analgesic response.[58]
Neuroendocrine disruption
Levels of hormones under the direct or indirect control of growth hormone (GH), including insulin-like growth factor 1 (IGF-1), cortisol, leptin and neuropeptide Y may be abnormal in people with fibromyalgia.[59][60] Several authors have demonstrated low growth hormone levels or low IGF-I levels in patients with fibromyalgia compared with controls. Moreover, people with fibromyalgia have an abnormal sleep pattern involving stages 3 and 4 of non-REM sleep during which growth hormone is predominantly secreted.[61] Further support for a causal role for growth hormone deficiency comes from observations that such deficiency in adults has been associated with many of the symptoms described by people with fibromyalgia. Growth hormone is important in maintaining muscle homeostasis, and it has been suggested that low levels may be responsible for delayed healing of muscle microtrauma in fibromyalgia.[62] Low (IGF-1) levels in some people with fibromyalgia have led to the theory that these people may actually have a different, treatable syndrome, adult growth hormone deficiency.[63] However, there remains some disagreement about the role of HGH in fibromyalgia.[64]
People with fibromyalgia may have alterations of normal neuroendocrine function, characterized by mild hypocortisolemia,[65] hyperreactivity of pituitary adrenocorticotropin hormone release in response to challenge, and glucocorticoid feedback resistance.[66]
Other abnormalities include reduced responsivity of thyrotropin and thyroid hormones to thyroid-releasing hormone,[67] a mild elevation of prolactin levels with disinhibition of prolactin release in response to challenge[68] and hyposecretion of adrenal androgens.[69]
These changes might result from chronic stress, which, after being perceived and processed by the central nervous system, activates hypothalamic corticotrophin-releasing hormone neurons. Chronic overactivity of these neurons could disrupt normal function of the pituitary-adrenal axis and cause an increased stimulation of hypothalamic somatostatin secretion, which, in turn, could inhibit the secretion of other hormones.[70]
Sympathetic hyperactivity
Functional analysis of the autonomic system in people with fibromyalgia has demonstrated disturbed activity characterized by hyperactivity of the sympathetic nervous system at baseline[71] with reduced sympathoadrenal reactivity in response to a variety of stressors including physical exertion and mental stress.[72][73] People with fibromyalgia demonstrate lower heart rate variability, an index of sympathetic/parasympathetic balance, indicating sustained sympathetic hyperactivity, especially at night.[74] In addition, plasma levels of neuropeptide Y, which is co-localized with norepinephrine in the sympathetic nervous system, have been reported as low in people with fibromyalgia,[60] while circulating levels of epinephrine and norepinephrine have been variously reported as low, normal and high.[75][76] Administration of interleukin-6, a cytokine capable of stimulating the release of hypothalamic corticotropin-releasing hormone which in turn stimulates activity within the sympathetic nervous system, results in a dramatic increase in circulating norepinephrine levels and a significantly greater increase in heart rate over baseline in people with fibromyalgia as compared to healthy controls.[77]
Cerebrospinal fluid
One of the most reproduced laboratory finding in people with fibromyalgia is an elevation in cerebrospinal fluid levels of substance P, a putative nociceptive neurotransmitter.[78][79] Metabolites for the monoamine neurotransmitters serotonin, norepinephrine, and dopamine – all of which play a role in natural analgesia – have been shown to be lower,[52] while concentrations of endogenous opioids (i.e., endorphins and enkephalins) appear to be higher.[80] The mean concentration of nerve growth factor, a substance known to participate in structural and functional plasticity of nociceptive pathways within the dorsal root ganglia and spinal cord, is elevated.[81] There is also evidence for increased excitatory amino acid release within cerebrospinal fluid, with a correlation demonstrated between levels for metabolites of glutamate and nitric oxide and clinical indices of pain.[82]
Brain imaging studies
Evidence of abnormal brain involvement in fibromyalgia has been provided via functional neuroimaging. The first findings reported were decreased blood flow within the thalamus and elements of the basal ganglia and mid-brain (i.e., pontine nucleus).[83][84] Differential activation in response to painful stimulation has also been demonstrated.[85][86] Brain centers showing hyperactivation in response to noxious stimulation include such pain-related brain centers as the primary and secondary somatosensory cortices, anterior cingulate cortex, and insular cortex. People also exhibit neural activation in brain regions associated with pain perception in response to nonpainful stimuli in such areas as the prefrontal, supplemental motor, insular, and cingulate cortices.
Evidence of hippocampal disruption indicated by reduced brain metabolite ratios has been demonstrated by studies using single-voxel magnetic resonance spectroscopy (1H-MRS).[34][35] A significant negative correlation was demonstrated between abnormal metabolite ratios and a validated index of the clinical severity (i.e. the Fibromyalgia Impact Questionnaire).[87] Correlations between clinical pain severity and concentrations of the excitatory amino acid neurotransmitter glutamate within the insular cortex have also been demonstrated using 1H-MRS.[88]
An acceleration of normal age-related brain atrophy has been demonstrated using voxel-based morphometry (VBM) with areas of reduced gray matter located in the cingulate cortex, insula and parahippocampal gyrus. Grey matter loss appears to increase 9.5 times the normal rate with each year.[89] Studies utilizing positron emission tomography have demonstrated reduced dopamine synthesis in the brainstem and elements of the limbic cortex.[90]
A significant negative correlation between pain severity and dopamine synthesis was demonstrated within the insular cortex. A subsequent study demonstrated gross disruption of dopaminergic reactivity in response to a tonic pain stimulus within the basal ganglia with a significant positive correlation between the defining feature of the disorder (i.e. tender point index) and dopamine D2 receptor binding potential specifically in the right putamen.[91]
Finally, reduced availability of mu-opioid receptors in the ventral striatum/nucleus accumbens and cingulate cortex has been demonstrated, with a significant negative correlation between affective pain levels and receptor availability in the nucleus accumbens.[92]
People with FMS consistently demonstrate altered activity in the insula, amygdala, anterior/mid cingulate cortex, superior temporal gyrus, the primary and secondary somatosensory cortex, and lingual gyrus. The changes show similarities to those found in other chronic pain conditions, but it is not known whether these specific features relate to all chronic pain or just to FMS.[93]
Diagnosis
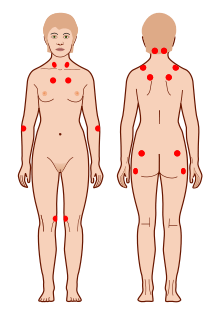
There is no single test that can fully diagnose fibromyalgia and there is debate over what should be considered essential diagnostic criteria and whether an objective diagnosis is possible. In most cases, people with fibromyalgia symptoms may also have laboratory test results that appear normal and many of their symptoms may mimic those of other rheumatic conditions such as arthritis or osteoporosis. The most widely accepted set of classification criteria for research purposes was elaborated in 1990 by the Multicenter Criteria Committee of the American College of Rheumatology. These criteria, which are known informally as "the ACR 1990", define fibromyalgia according to the presence of the following criteria:
- A history of widespread pain lasting more than three months – affecting all four quadrants of the body, i.e., both sides, and above and below the waist.
- Tender points – there are 18 designated possible tender points (although a person with the disorder may feel pain in other areas as well). Diagnosis is no longer based on the number of tender points.[94][95]
The ACR criteria for the classification of patients were originally established as inclusion criteria for research purposes and were not intended for clinical diagnosis but have now become the de facto diagnostic criteria in the clinical setting. It should be noted that the number of tender points that may be active at any one time may vary with time and circumstance. A controversial study was done by a legal team looking to prove their client's disability based primarily on tender points and their widespread presence in non-litigious communities prompted the lead author of the ACR criteria to question now the useful validity of tender points in diagnosis.[96] Use of control points has been used to cast doubt on whether a person has fibromyalgia, and to claim the person is malingering; however, no research has been done for the use of control points to diagnose fibromyalgia, and such diagnostic tests have been advised against, and people complaining of pain all over should still have fibromyalgia considered as a diagnosis.[9]
2010 provisional criteria
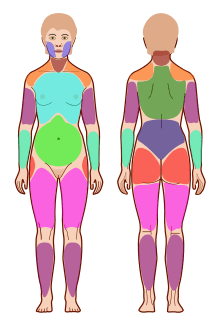
In 2010, the American College of Rheumatology approved provisional revised diagnostic criteria for fibromyalgia that eliminated the 1990 criteria's reliance on tender point testing.[97] The revised criteria use a widespread pain index (WPI) and symptom severity scale (SS) in place of tender point testing under the 1990 criteria. The WPI counts up to 19 general body areas[lower-alpha 1] in which the person has experienced pain in the preceding two weeks. The SS rates the severity of the person's fatigue, unrefreshed waking, cognitive symptoms, and general somatic symptoms,[lower-alpha 2] each on a scale from 0 to 3, for a composite score ranging from 0 to 12. The revised criteria for diagnosis are:
- WPI ≥ 7 and SS ≥ 5 OR WPI 3–6 and SS ≥ 9,
- Symptoms have been present at a similar level for at least three months, and
- No other diagnosable disorder otherwise explains the pain.[97]:607
Differential diagnosis
Two patients initially diagnosed with fibromyalgia were subsequently shown to have myotonia congenita.[98] Both patients had been seen by rheumatologists and diagnosed with fibromyalgia. The diagnoses were revised when electromyographic studies revealed myotonic discharges in some of the muscles. Genetic analysis of the skeletal muscle chloride channel which is the underlying cause of myotonia congenita revealed that both patients had mutations in this gene. Over 130 mutations in this gene have been described and the phenotype is very variable which may account for the difficulty in the initial diagnosis.[99][100]
Management
As with many other medically unexplained syndromes, there is no universally accepted treatment or cure for fibromyalgia, and treatment typically consists of symptom management. Developments in the understanding of the pathophysiology of the disorder have led to improvements in treatment, which include prescription medication, behavioral intervention, and exercise. Indeed, integrated treatment plans that incorporate medication, patient education, aerobic exercise and cognitive behavioral therapy have been shown to be effective in alleviating pain and other fibromyalgia-related symptoms.[101]
The Association of the Scientific Medical Societies in Germany,[102] the European League Against Rheumatism[103] and the Canadian Pain Society[104] currently publish guidelines for the diagnosis and management of FMS.
Medications
Health Canada and the US Food and Drug Administration (FDA) have approved pregabalin[105] and duloxetine, for the management of fibromyalgia. The FDA also approves milnacipran, but the European Medicines Agency refused marketing authority.[106]
Antidepressants
Antidepressants are "associated with improvements in pain, depression, fatigue, sleep disturbances, and health-related quality of life in people with FMS."[107] The goal of antidepressants should be symptom reduction and if used long term, their effects should be evaluated against side effects. A small number of people benefit significantly from the SNRIs duloxetine and milnacipran and the tricyclic antidepressants (TCAs), such as amitriptyline. However many people experience more adverse effects than benefits.[108][109] While amitriptyline has been used as a first line treatment, the quality of evidence to support this use is poor.[110]
It can take up to three months to derive benefit from the antidepressant amitriptyline and up to six months to gain the maximal response from duloxetine, milnacipran, and pregabalin. Some medications have the potential to cause withdrawal symptoms when stopping so gradual discontinuation may be warranted particularly for antidepressants and pregabalin.[9]
SSRIs and (TCAs) have more favorable response rates and fewer side effects compared to Pregabalin and the SNRIs Duloxetine and Milnacipram, making them the most effect agents for treating fibromyalgia.[111]
Anti-seizure medication
The anti-convulsant drugs gabapentin[112] and pregabalin can be used to reduce pain.[113] Gabapentin is of a significant benefit in about 30% of people who take it. However, it is commonly associated with adverse effects.[112] Pregabalin demonstrates a substantial benefit in about 9% of people.[114] Pregabalin reduced time off work by 0.2 days per week.[115]
Opioids
The use of opioids is controversial. As of 2015 no opioid is approved for use in this condition by the FDA.[116] The National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) in 2014 stated that there was a lack of evidence for opioids.[4] The Association of the Scientific Medical Societies in Germany in 2012 made no recommendation either for or against the use of weak opioids because of the limited amount of scientific research addressing their use in the treatment of FM. They strongly advise against using strong opioids.[102] The Canadian Pain Society in 2012 said that opioids, starting with a weak opioid like tramadol, can be tried but only for people with moderate to severe pain that is not well-controlled by non-opioid painkillers. They discourage the use of strong opioids and only recommend using them while they continue to provide improved pain and functioning. Healthcare providers should monitor people on opioids for ongoing effectiveness, side effects and possible unwanted drug behaviors.[104]
The European League Against Rheumatism in 2008 recommends tramadol and other weak opioids may be used for pain but not strong opioids.[103] A 2015 review found fair evidence to support tramadol use if other medications do not work.[116] Goldenberg et al suggest that tramadol works via its serotonin and norepinephrine reuptake inhibition, rather than via its action as a weak opioid receptor agonist.[7] The combination of tramadol and paracetemol has demonstrated efficacy, safety and tolerability (for up to two years in the management of other pain conditions) without the development of tolerance. It is as effective as a combination of codeine (another mild opioid) and paracetamol but produces less sleepiness and constipation.[117]
A large study of US people with fibromyalgia found that between 2005 and 2007 37.4% were prescribed short-acting opioids and 8.3% were prescribed long-acting opioids,[118] with around 10% of those prescribed short-acting opioids using tramadol;[119] and a 2011 Canadian study of 457 people with FM found 32% used opioids and two thirds of those used strong opioids.[104]
Others
A 2007 review of three randomized placebo controlled studies concluded that a period of nine months of growth hormone was required to reduce fibromyalgia symptoms and normalize IGF-1.[120] A 2014 also found some evidence support its use.[121] Sodium oxybate increases growth hormone production levels through increased slow-wave sleep patterns. However, this medication was not approved by the FDA for the indication for use in people with fibromyalgia due to the concern for abuse.[122]
The muscle relaxants cyclobenzaprine and tizanidine are sometimes used off-label to treat fibromyalgia.[123] The use of NSAIDs is not recommended as first line therapy.[124]
Dopamine agonists (e.g. pramipexole and ropinirole) resulted in some improvement in a minority of people,[48] but numerous side effects, including the onset of impulse control disorders like compulsive gambling and shopping, have led to concern about this approach.[125]
There is some evidence that 5HT3 antagonists may be beneficial.[126] Preliminary clinical data finds that low-dose naltrexone (LDN) may provide symptomatic improvement.[127]
Therapy
Due to the uncertainty about the pathogenesis of FM, current treatment approaches focus on management of symptoms to improve quality of life,[128] using integrated pharmacological and non-pharmacological approaches.[129] There is no single intervention shown to be effective for all patients [130] and no gold treatment standard exists for FM.[131] Multimodal/multidisciplinary therapy is recommended to target multiple underlying factors of FM.[132] A meta-analysis of 1,119 subjects found "strong evidence that multicomponent treatment has beneficial short-term effects on key symptoms of FMS." [133]
Cognitive behavioural therapy
Non-pharmacological components include cognitive-behavioural therapy (CBT), exercise and psychoeducation (specifically, sleep hygiene).[134][135][136][137] CBT and related psychological and behavioural therapies have a small to moderate effect in reducing symptoms of fibromyalgia.[138][139] Effect sizes tend to be small when CBT is used as a stand-alone treatment for FM patients, but these improve significantly when CBT is part of a wider multidisciplinary treatment program.[140] The greatest benefit occurs when CBT is used along with exercise.[101][141]
A 2010 systematic review of 14 studies reported that CBT improves self-efficacy or coping with pain and reduces the number of physician visits at post-treatment, but has no significant effect on pain, fatigue, sleep or health-related quality of life at post-treatment or follow-up. Depressed mood was also improved but this could not be distinguished from some risks of bias.[142]
Exercise
Exercise improves fitness and sleep and may reduce pain and fatigue in some people with fibromyalgia.[143][144] In particular, there is strong evidence that cardiovascular exercise is effective for some people.[145] Long-term aquatic-based exercise has been proven beneficial as it combines cardiovascular exercise with resistance training.[146]
In children, fibromyalgia is often treated with an intense physical and occupational therapy program for amplified musculoskeletal pain syndromes. These programs also employ counseling, art therapy, and music therapy. These programs are evidence-based and report long-term total pain resolution rates as high as 88%.[147]
Prognosis
Although in itself neither degenerative nor fatal, the chronic pain of fibromyalgia is pervasive and persistent. Most people with fibromyalgia report that their symptoms do not improve over time. An evaluation of 332 consecutive new people with fibromyalgia found that disease-related factors such as pain and psychological factors such as work status, helplessness, education, and coping ability had an independent and significant relationship to FM symptom severity and function.[148]
Epidemiology
Fibromyalgia is estimated to affect 2–8% of the population,[3][149] with a female to male incidence ratio that is somewhere between 7:1 and 9:1.[14][150]
Fibromyalgia may not be diagnosed in up to 75% of affected people.[12]
History
Chronic widespread pain had already been described in the literature in the 19th century but the term fibromyalgia was not used until 1976 when Dr P.K. Hench used it to describe these symptoms.[9] Many names, including "muscular rheumatism", "fibrositis", "psychogenic rheumatism", and "neurasthenia" were applied historically to symptoms resembling those of fibromyalgia.[151] The term fibromyalgia was coined by researcher Mohammed Yunus as a synonym for fibrositis and was first used in a scientific publication in 1981.[152] Fibromyalgia is from the Latin fibra (fiber)[153] and the Greek words myo (muscle)[154] and algos (pain).[155]
Historical perspectives on the development of the fibromyalgia concept note the "central importance" of a 1977 paper by Smythe and Moldofsky on fibrositis.[156][157] The first clinical, controlled study of the characteristics of fibromyalgia syndrome was published in 1981,[158] providing support for symptom associations. In 1984, an interconnection between fibromyalgia syndrome and other similar conditions was proposed,[159] and in 1986, trials of the first proposed medications for fibromyalgia were published.[159]
A 1987 article in the Journal of the American Medical Association used the term "fibromyalgia syndrome" while saying it was a "controversial condition".[160] The American College of Rheumatology (ACR) published its first classification criteria for fibromyalgia in 1990,[161] although these are not strictly diagnostic criteria.[13]
Society and culture
Economics
People with fibromyalgia generally have higher health care costs and utilization rates. A study of almost 20,000 Humana members enrolled in Medicare Advantage and commercial plans compared costs and medical utilizations and found that people with fibromyalgia used twice as much pain-related medication as those without fibromyalgia. Furthermore, the use of medications and medical necessities increased markedly across many measures once diagnosis was made.[162]
Controversies
Being a disorder defined relatively recently and still not completely understood, fibromyalgia continues to be a diagnosis that sometimes is disputed. Dr. Frederick Wolfe, lead author of the 1990 paper that first defined the diagnostic guidelines for fibromyalgia, stated in 2008, that he believed it "clearly" not to be a disease but instead a physical response to depression and stress,[163] and in 2013 that its causes "are controversial in a sense" and "there are many factors that produce these symptoms – some are psychological and some are physical and it does exist on a continuum".[164]
Some members of the medical community do not consider fibromyalgia a disease because of a lack of abnormalities on physical examination and the absence of objective diagnostic tests.[156][165] Yunus objects to the psychological characterization of FM. He argues that data indicating it is not psychological has been ignored or manipulated.[166]
Neurologists and pain specialists tend to view fibromyalgia as a pathology due to dysfunction of muscles and connective tissue as well as functional abnormalities in the central nervous system. Rheumatologists define the syndrome in the context of "central sensitization" – heightened brain response to normal stimuli in the absence of disorders of the muscles, joints, or connective tissues. On the other hand, psychiatrists often view fibromyalgia as a type of affective disorder, whereas specialists in psychosomatic medicine tend to view fibromyalgia as being a somatic symptom disorder. However, there is extensive research evidence to support the view that the central symptom of fibromyalgia, namely pain, has a neurogenic origin. These controversies don't engage healthcare specialists alone; some patients object to fibromyalgia being described in purely somatic terms.[9][12]
The validity of fibromyalgia as a unique clinical entity is a matter of contention because "no discrete boundary separates syndromes such as FMS, chronic fatigue syndrome, irritable bowel syndrome, or chronic muscular headaches".[145][167] Because of this symptomatic overlap, some researchers have proposed that fibromyalgia and other analogous syndromes be classified together as functional somatic syndromes for some purposes.[168]
Research
Investigational medications include cannabinoids and the 5-HT3 receptor antagonist tropisetron.[169] Low quality evidence found an improvement in symptoms with a gluten free diet among those without celiac disease.[170] A controlled study of guaifenesin failed to demonstrate any benefits from this treatment.[171][172]
Notes
- ↑ Shoulder girdle (left & right), upper arm (left & right), lower arm (left & right), hip/buttock/trochanter (left & right), upper leg (left & right), lower leg (left & right), jaw (left & right), chest, abdomen, back (upper & lower), and neck.[97]:607
- ↑ Somatic symptoms include, but are not limited to: muscle pain, irritable bowel syndrome, fatigue or tiredness, problems thinking or remembering, muscle weakness, headache, pain or cramps in the abdomen, numbness or tingling, dizziness, insomnia, depression, constipation, pain in the upper abdomen, nausea, nervousness, chest pain, blurred vision, fever, diarrhea, dry mouth, itching, wheezing, Raynaud's phenomenon, hives or welts, ringing in the ears, vomiting, heartburn, oral ulcers, loss of or changes in taste, seizures, dry eyes, shortness of breath, loss of appetite, rash, sun sensitivity, hearing difficulties, easy bruising, hair loss, frequent or painful urination, and bladder spasms.[97]:607
References
- ↑ "fibromyalgia". Collins Dictionaries. Retrieved 16 March 2016.
- 1 2 3 Ngian GS, Guymer EK, Littlejohn GO (February 2011). "The use of opioids in fibromyalgia". Int J Rheum Dis. 14 (1): 6–11. doi:10.1111/j.1756-185X.2010.01567.x. PMID 21303476.
- 1 2 3 4 5 6 7 8 9 10 Clauw, Daniel J. (16 April 2014). "Fibromyalgia". JAMA. 311 (15): 1547–55. doi:10.1001/jama.2014.3266. PMID 24737367.
- 1 2 3 4 5 6 7 8 9 10 "Questions and Answers about Fibromyalgia". NIAMS. July 2014. Retrieved 15 March 2016.
- 1 2 Buskila D, Sarzi-Puttini P (2006). "Biology and therapy of fibromyalgia. Genetic aspects of fibromyalgia syndrome". Arthritis Research & Therapy. 8 (5): 218. doi:10.1186/ar2005. PMC 1779444
 . PMID 16887010.
. PMID 16887010. - ↑ "Fibromyalgia". American College of Rheumatology. May 2015. Retrieved 16 March 2016.
- 1 2 Goldenberg, DL; Clauw, DJ; Palmer, RE; Clair, AG (May 2016). "Opioid Use in Fibromyalgia: A Cautionary Tale.". Mayo Clinic Proceedings (Review). 91 (5): 640–8. doi:10.1016/j.mayocp.2016.02.002. PMID 26975749.
- ↑ Sumpton, JE; Moulin, DE (2014). "Fibromyalgia.". Handbook of clinical neurology. 119: 513–27. doi:10.1016/B978-0-7020-4086-3.00033-3. PMID 24365316.
- 1 2 3 4 5 6 7 8 Häuser W, Eich W, Herrmann M, Nutzinger DO, Schiltenwolf M, Henningsen P (June 2009). "Fibromyalgia syndrome: classification, diagnosis, and treatment". Dtsch Arztebl Int. 106 (23): 383–91. doi:10.3238/arztebl.2009.0383. PMC 2712241
 . PMID 19623319.
. PMID 19623319. - ↑ Wang, SM; Han, C; Lee, SJ; Patkar, AA; Masand, PS; Pae, CU (June 2015). "Fibromyalgia diagnosis: a review of the past, present and future.". Expert Review of Neurotherapeutics. 15 (6): 667–79. doi:10.1586/14737175.2015.1046841. PMID 26035624.
- ↑ Bergmann, Uri (2012). Neurobiological foundations for EMDR practice. New York, NY: Springer Pub. Co. p. 165. ISBN 9780826109385.
- 1 2 3 4 Clauw DJ, Arnold LM, McCarberg BH (September 2011). "The science of fibromyalgia". Mayo Clin Proc. 86 (9): 907–11. doi:10.4065/mcp.2011.0206. PMC 3258006
 . PMID 21878603.
. PMID 21878603. - 1 2 Müller W, Schneider EM, Stratz T (September 2007). "The classification of fibromyalgia syndrome". Rheumatol Int. 27 (11): 1005–10. doi:10.1007/s00296-007-0403-9. PMID 17653720.
- 1 2 3 4 5 Hawkins RA (September 2013). "Fibromyalgia: A Clinical Update". Journal of the American Osteopathic Association. 113 (9): 680–689. doi:10.7556/jaoa.2013.034. PMID 24005088.
- ↑ "Information on Fibromyalgia". Healthline.com. 21 August 2012. Retrieved 26 August 2012.
- ↑ Wallace DJ, Hallegua DS (October 2002). "Fibromyalgia: the gastrointestinal link". Curr Pain Headache Rep. 8 (5): 364–8. doi:10.1007/s11916-996-0009-z. PMID 15361320.
- 1 2 3 Moldofsky H, Scarisbrick P, England R, Smythe H (1975). "Musculoskeletal symptoms and non-REM sleep disturbance in patients with "fibrositis syndrome" and healthy subjects". Psychosom Med. 37 (4): 341–51. doi:10.1097/00006842-197507000-00008. PMID 169541. Retrieved 21 May 2008.
- ↑ Glass JM (December 2006). "Cognitive dysfunction in fibromyalgia and chronic fatigue syndrome: new trends and future directions". Curr Rheumatol Rep. 8 (6): 425–9. doi:10.1007/s11926-006-0036-0. PMID 17092441.
- 1 2 3 4 Leavitt F, Katz RS, Mills M, Heard AR (2002). "Cognitive and Dissociative Manifestations in Fibromyalgia". J Clin Rheumatol. 8 (2): 77–84. doi:10.1097/00124743-200204000-00003. PMID 17041327.
- 1 2 3 4 5 Buskila D, Cohen H (October 2007). "Comorbidity of fibromyalgia and psychiatric disorders". Curr Pain Headache Rep. 11 (5): 333–8. doi:10.1007/s11916-007-0214-4. PMID 17894922.
- ↑ Yunus MB (June 2007). "Role of central sensitization in symptoms beyond muscle pain, and the evaluation of a patient with widespread pain". Best Pract Res Clin Rheumatol. 21 (3): 481–97. doi:10.1016/j.berh.2007.03.006. PMID 17602995.
- ↑ Maletic V, Raison CL (2009). "Neurobiology of depression, fibromyalgia and neuropathic pain". Front Biosci. 14: 5291–338. doi:10.2741/3598. PMID 19482616.
- ↑ Cohen H, Buskila D, Neumann L, Ebstein RP (March 2002). "Confirmation of an association between fibromyalgia and serotonin transporter promoter region (5- HTTLPR) polymorphism, and relationship to anxiety-related personality traits". Arthritis Rheum. 46 (3): 845–7. doi:10.1002/art.10103. PMID 11920428.
- ↑ Buskila D, Dan B, Cohen H, Hagit C, Neumann L, Lily N, Ebstein RP (August 2004). "An association between fibromyalgia and the dopamine D4 receptor exon III repeat polymorphism and relationship to novelty seeking personality traits". Mol. Psychiatry. 9 (8): 730–1. doi:10.1038/sj.mp.4001506. PMID 15052273.
- ↑ Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D (February 2003). "COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor". Science. 299 (5610): 1240–3. doi:10.1126/science.1078546. PMID 12595695.
- ↑ Narita M, Nishigami N, Narita N, Yamaguti K, Okado N, Watanabe Y, Kuratsune H (November 2003). "Association between serotonin transporter gene polymorphism and chronic fatigue syndrome". Biochem. Biophys. Res. Commun. 311 (2): 264–6. doi:10.1016/j.bbrc.2003.09.207. PMID 14592408.
- ↑ Camilleri M, Atanasova E, Carlson PJ, Ahmad U, Kim HJ, Viramontes BE, McKinzie S, Urrutia R (August 2002). "Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome". Gastroenterology. 123 (2): 425–32. doi:10.1053/gast.2002.34780. PMID 12145795.
- ↑ Hudson JI, Mangweth B, Pope HG, De Col C, Hausmann A, Gutweniger S, Laird NM, Biebl W, Tsuang MT (February 2003). "Family study of affective spectrum disorder". Arch. Gen. Psychiatry. 60 (2): 170–7. doi:10.1001/archpsyc.60.2.170. PMID 12578434.
- ↑ Lee YH, Choi SJ, Ji JD, Song GG (February 2012). "Candidate gene studies of fibromyalgia: a systematic review and meta-analysis". Rheumatol. Int. 32 (2): 417–26. doi:10.1007/s00296-010-1678-9. PMID 21120487.
- ↑ Anderberg UM, Marteinsdottir I, Theorell T, von Knorring L (August 2000). "The impact of life events in female patients with fibromyalgia and in female healthy controls". Eur Psychiatry. 15 (5): 33–41. doi:10.1016/S0924-9338(00)00397-7. PMID 10954873.
- 1 2 Schweinhardt P, Sauro KM, Bushnell MC (October 2008). "Fibromyalgia: a disorder of the brain?". Neuroscientist. 14 (5): 415–21. doi:10.1177/1073858407312521. PMID 18270311.
- ↑ Häuser W, Kosseva M, Üceyler N, Klose P, Sommer C (2011). "Emotional, physical, and sexual abuse in fibromyalgia syndrome: A systematic review with meta-analysis". Arthritis Care & Research. 63 (6): 808–820. doi:10.1002/acr.20328. PMID 20722042.
- ↑ Sommer C, Häuser W, Burgmer M, Engelhardt R, Gerhold K, Petzke F, Schmidt-Wilcke T, Späth M, Tölle T, Uçeyler N, Wang H, Winkelmann A, Thieme K (June 2012). "[Etiology and pathophysiology of fibromyalgia syndrome]". Schmerz. 26 (3): 259–67. doi:10.1007/s00482-012-1174-0. PMID 22760458.
- 1 2 Emad Y, Ragab Y, Zeinhom F, El-Khouly G, Abou-Zeid A, Rasker JJ (July 2008). "Hippocampus dysfunction may explain symptoms of fibromyalgia syndrome. A study with single-voxel magnetic resonance spectroscopy". J. Rheumatol. 35 (7): 1371–7. PMID 18484688.
- 1 2 Wood PB, Ledbetter CR, Glabus MF, Broadwell LK, Patterson JC (2008). "Hippocampal Metabolite Abnormalities in Fibromyalgia: Correlation With Clinical Features". J Pain. 10 (1): 47–52. doi:10.1016/j.jpain.2008.07.003. PMID 18771960.
- ↑ McBeth J, Chiu YH, Silman AJ, Ray D, Morriss R, Dickens C, Gupta A, Macfarlane GJ (2005). "Hypothalamic-pituitary-adrenal stress axis function and the relationship with chronic widespread pain and its antecedents". Arthritis Research & Therapy. 7 (5): R992–R1000. doi:10.1186/ar1772. PMC 1257426
 . PMID 16207340.
. PMID 16207340. - ↑ Moldofsky H, Scarisbrick P (January–February 1976). "Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation". Psychosom Med. 38 (1): 35–44. doi:10.1097/00006842-197601000-00006. PMID 176677.
- ↑ Goldenberg DL (April 1999). "Fibromyalgia syndrome a decade later: what have we learned?". Arch. Intern. Med. 159 (8): 777–85. doi:10.1001/archinte.159.8.777. PMID 10219923.
- ↑ Geoffroy PA, Amad A, Gangloff C, Thomas P (May 2012). "Fibromyalgia and psychiatry: 35 years later… what's new?". Presse Med. 41 (5): 555–65. doi:10.1016/j.lpm.2011.08.008. PMID 21993145.
- ↑ Pae CU, Luyten P, Marks DM, Han C, Park SH, Patkar AA, Masand PS, Van Houdenhove B (August 2008). "The relationship between fibromyalgia and major depressive disorder: a comprehensive review". Curr Med Res Opin. 24 (8): 2359–71. doi:10.1185/03007990802288338. PMID 18606054.
- ↑ Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ (May 2005). "The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort". Arthritis Rheum. 52 (5): 1577–84. doi:10.1002/art.21008. PMID 15880832.
- ↑ Alciati A, Sarzi-Puttini P, Batticiotto A, Torta R, Gesuele F, Atzeni F, Angst J (2012). "Overactive lifestyle in patients with fibromyalgia as a core feature of bipolar spectrum disorder". Clinical and Experimental Rheumatology. 30 (6): 122–128. PMID 23261011.
- ↑ Rossi A, Di Lollo AC, Guzzo MP, Giacomelli C, Atzeni F, Bazzichi L, Di Franco M (2015). "Fibromyalgia and nutrition: what news?". Clin Exp Rheumatol. 33 (1 Suppl 88): S117–25. PMID 25786053.
- ↑ San Mauro Martín I, Garicano Vilar E, Collado Yurrutia L, Ciudad Cabañas MJ (Dec 2014). "[Is gluten the great etiopathogenic agent of disease in the XXI century?] [Article in Spanish]" (PDF). Nutr Hosp. 30 (6): 1203–10. doi:10.3305/nh.2014.30.6.7866. PMID 25433099.
- ↑ Wood PB (2004). "Stress and dopamine: implications for the pathophysiology of chronic widespread pain". Medical Hypotheses. 62 (3): 420–424. doi:10.1016/j.mehy.2003.10.013. PMID 14975515.
- ↑ Cervenka S, Pålhagen SE, Comley RA, Panagiotidis G, Cselényi Z, Matthews JC, Lai RY, Halldin C, Farde L (August 2006). "Support for dopaminergic hypoactivity in restless legs syndrome: a PET study on D2-receptor binding". Brain. 129 (Pt 8): 2017–28. doi:10.1093/brain/awl163. PMID 16816393.
- ↑ Yunus MB, Aldag JC (May 1996). "Restless legs syndrome and leg cramps in fibromyalgia syndrome: a controlled study". BMJ. 312 (7042): 1339. doi:10.1136/bmj.312.7042.1339. PMC 2351040
 . PMID 8646049. Retrieved 21 May 2008.
. PMID 8646049. Retrieved 21 May 2008. - 1 2 Holman AJ, Myers RR (August 2005). "A randomized, double-blind, placebo-controlled trial of pramipexole, a dopamine agonist, in patients with fibromyalgia receiving concomitant medications". Arthritis & Rheumatism. 52 (8): 2495–505. doi:10.1002/art.21191. PMID 16052595.
- ↑ Bradley, Laurence A. (1 December 2016). "Pathophysiology of Fibromyalgia". The American journal of medicine. 122 (12 Suppl): S22. doi:10.1016/j.amjmed.2009.09.008. ISSN 0002-9343.
- ↑ Russell IJ, Michalek JE, Vipraio GA, Fletcher EM, Javors MA, Bowden CA (January 1992). "Platelet 3H-imipramine uptake receptor density and serum serotonin levels in patients with fibromyalgia/fibrositis syndrome". Journal of Rheumatology. 19 (1): 104–9. PMID 1313504.
- ↑ Bradley, Laurence A. (1 December 2016). "Pathophysiology of Fibromyalgia". The American journal of medicine. 122 (12 Suppl): S22. doi:10.1016/j.amjmed.2009.09.008. ISSN 0002-9343.
- 1 2 Russell IJ, Vaeroy H, Javors M, Nyberg F (May 1992). "Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis". Arthritis Rheum. 35 (5): 550–6. doi:10.1002/art.1780350509. PMID 1374252.
- ↑ Arnold LM (2006). "Biology and therapy of fibromyalgia. New therapies in fibromyalgia". Arthritis Research & Therapy. 8 (4): 212. doi:10.1186/ar1971. PMC 1779399
 . PMID 16762044.
. PMID 16762044. - ↑ Jaschko G, Hepp U, Berkhoff M, Schmet M, Michel BA, Gay S, Sprott H (September 2007). "Serum serotonin levels are not useful in diagnosing fibromyalgia". Ann. Rheum. Dis. 66 (9): 1267–8. doi:10.1136/ard.2006.058842. PMC 1955138
 . PMID 17693607.
. PMID 17693607. - ↑ Späth M (May 2002). "Current experience with 5-HT3 receptor antagonists in fibromyalgia". Rheum Dis Clin North Am. 28 (2): 319–28. doi:10.1016/S0889-857X(01)00014-X. PMID 12122920.
- ↑ Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, Dayer P, Vischer TL (May 2003). "Neurophysiologic evidence for a central sensitization in patients with fibromyalgia". Arthritis Rheum. 48 (5): 1420–9. doi:10.1002/art.10893. PMID 12746916.
- ↑ Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD (March 2001). "Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome". Pain. 91 (1–2): 165–75. doi:10.1016/S0304-3959(00)00432-2. PMID 11240089.
- ↑ Staud R, Robinson ME, Price DD (November 2005). "Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls". Pain. 118 (1–2): 176–84. doi:10.1016/j.pain.2005.08.007. PMID 16154700.
- ↑ Bellato, Enrico; Marini, Eleonora; Castoldi, Filippo; Barbasetti, Nicola; Mattei, Lorenzo; Bonasia, Davide Edoardo; Blonna, Davide (1 January 2012). "Fibromyalgia Syndrome: Etiology, Pathogenesis, Diagnosis, and Treatment". Pain Research and Treatment. 2012. doi:10.1155/2012/426130. ISSN 2090-1542.
- 1 2 Anderberg UM, Liu Z, Berglund L, Nyberg F (1999). "Elevated plasma levels of neuropeptide Y in female fibromyalgia patients". Eur J Pain. 3 (1): 19–30. doi:10.1016/S1090-3801(99)90185-4. PMID 10700334.
- ↑ Bennett, Robert. "Growth Hormone Deficiency in Fibromyalgia". Retrieved 17 August 2013.
- ↑ Gupta & Silman. "Psychological stress and fibromyalgia: a review of the evidence suggesting a neuroendocrine link". Arthritis Research & Therapy. Retrieved 9 August 2013.
- ↑ Bennett RM (August 2002). "Adult growth hormone deficiency in patients with fibromyalgia". Curr Rheumatol Rep. 4 (4): 306–12. doi:10.1007/s11926-002-0039-4. PMID 12126582.
- ↑ Shuer ML (2003). "Fibromyalgia: symptom constellation and potential therapeutic options". Endocrine. 22 (1): 67–76. doi:10.1385/ENDO:22:1:67. PMID 14610300.
- ↑ Gur A, Cevik R, Sarac AJ, Colpan L, Em S (November 2004). "Hypothalamic-pituitary-gonadal axis and cortisol in young women with primary fibromyalgia: the potential roles of depression, fatigue, and sleep disturbance in the occurrence of hypocortisolism". Ann. Rheum. Dis. 63 (11): 1504–6. doi:10.1136/ard.2003.014969. PMC 1754816
 . PMID 15479904.
. PMID 15479904. - ↑ Griep EN, Boersma JW, Lentjes EG, Prins AP, van der Korst JK, de Kloet ER (July 1998). "Function of the hypothalamic-pituitary-adrenal axis in patients with fibromyalgia and low back pain". J. Rheumatol. 25 (7): 1374–81. PMID 9676772.
- ↑ Neeck G, Riedel W (July 1992). "Thyroid function in patients with fibromyalgia syndrome". J. Rheumatol. 19 (7): 1120–2. PMID 1512769.
- ↑ Riedel W, Layka H, Neeck G (1998). "Secretory pattern of GH, TSH, thyroid hormones, ACTH, cortisol, FSH, and LH in patients with fibromyalgia syndrome following systemic injection of the relevant hypothalamic-releasing hormones". Z Rheumatol. 57 Suppl 2 (8): 81–7. doi:10.1007/s003930050242. PMID 10025090.
- ↑ Dessein PH, Shipton EA, Joffe BI, Hadebe DP, Stanwix AE, Van der Merwe BA (November 1999). "Hyposecretion of adrenal androgens and the relation of serum adrenal steroids, serotonin and insulin-like growth factor-1 to clinical features in women with fibromyalgia". Pain. 83 (2): 313–9. doi:10.1016/S0304-3959(99)00113-X. PMID 10534604.
- ↑ Neeck G, Crofford LJ (November 2000). "Neuroendocrine perturbations in fibromyalgia and chronic fatigue syndrome". Rheum. Dis. Clin. North Am. 26 (4): 989–1002. doi:10.1016/S0889-857X(05)70180-0. PMID 11084955.
- ↑ Martinez-Lavin M (2007). "Biology and therapy of fibromyalgia. Stress, the stress response system, and fibromyalgia". Arthritis Research & Therapy. 9 (4): 216. doi:10.1186/ar2146. PMC 2206360
 . PMID 17626613.
. PMID 17626613. - ↑ Giske L, Vøllestad NK, Mengshoel AM, Jensen J, Knardahl S, Røe C (April 2008). "Attenuated adrenergic responses to exercise in women with fibromyalgia – A controlled study". Eur J Pain. 12 (3): 351–60. doi:10.1016/j.ejpain.2007.07.007. PMID 17827042.
- ↑ Nilsen KB, Sand T, Westgaard RH, Stovner LJ, White LR, Bang Leistad R, Helde G, Rø M (October 2007). "Autonomic activation and pain in response to low-grade mental stress in fibromyalgia and shoulder/neck pain patients". Eur J Pain. 11 (7): 743–55. doi:10.1016/j.ejpain.2006.11.004. PMID 17224287.
- ↑ Martínez-Lavín M, Hermosillo AG, Mendoza C, Ortiz R, Cajigas JC, Pineda C, Nava A, Vallejo M (April 1997). "Orthostatic sympathetic derangement in subjects with fibromyalgia". J. Rheumatol. 24 (4): 714–8. PMID 9101507.
- ↑ van Denderen JC, Boersma JW, Zeinstra P, Hollander AP, van Neerbos BR (1992). "Physiological effects of exhaustive physical exercise in primary fibromyalgia syndrome (PFS): is PFS a disorder of neuroendocrine reactivity?". Scand. J. Rheumatol. 21 (1): 35–7. doi:10.3109/03009749209095060. PMID 1570485.
- ↑ Adler GK, Kinsley BT, Hurwitz S, Mossey CJ, Goldenberg DL (May 1999). "Reduced hypothalamic-pituitary and sympathoadrenal responses to hypoglycemia in women with fibromyalgia syndrome". Am J Med. 106 (5): 534–43. doi:10.1016/S0002-9343(99)00074-1. PMID 10335725.
- ↑ Torpy DJ, Papanicolaou DA, Lotsikas AJ, Wilder RL, Chrousos GP, Pillemer SR (April 2000). "Responses of the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis to interleukin-6: a pilot study in fibromyalgia". Arthritis Rheum. 43 (4): 872–80. doi:10.1002/1529-0131(200004)43:4<872::AID-ANR19>3.0.CO;2-T. PMID 10765933.
- ↑ Russell IJ, Orr MD, Littman B, Vipraio GA, Alboukrek D, Michalek JE, Lopez Y, MacKillip F (November 1994). "Elevated cerebrospinal fluid levels of substance P in patients with the fibromyalgia syndrome". Arthritis Rheum. 37 (11): 1593–601. doi:10.1002/art.1780371106. PMID 7526868.
- ↑ Vaerøy H, Helle R, Førre O, Kåss E, Terenius L (January 1988). "Elevated CSF levels of substance P and high incidence of Raynaud phenomenon in patients with fibromyalgia: new features for diagnosis". Pain. 32 (1): 21–6. doi:10.1016/0304-3959(88)90019-X. PMID 2448729.
- ↑ Vaerøy H, Nyberg F, Terenius L (August 1991). "No evidence for endorphin deficiency in fibromyalgia following investigation of cerebrospinal fluid (CSF) dynorphin A and Met-enkephalin-Arg6-Phe7". Pain. 46 (2): 139–43. doi:10.1016/0304-3959(91)90068-9. PMID 1684241.
- ↑ Giovengo SL, Russell IJ, Larson AA (July 1999). "Increased concentrations of nerve growth factor in cerebrospinal fluid of patients with fibromyalgia". J. Rheumatol. 26 (7): 1564–9. PMID 10405946.
- ↑ Larson AA, Giovengo SL, Russell IJ, Michalek JE (August 2000). "Changes in the concentrations of amino acids in the cerebrospinal fluid that correlate with pain in patients with fibromyalgia: implications for nitric oxide pathways". Pain. 87 (2): 201–11. doi:10.1016/S0304-3959(00)00284-0. PMID 10924813.
- ↑ Mountz JM, Bradley LA, Modell JG, Alexander RW, Triana-Alexander M, Aaron LA, Stewart KE, Alarcón GS, Mountz JD (July 1995). "Fibromyalgia in women. Abnormalities of regional cerebral blood flow in the thalamus and the caudate nucleus are associated with low pain threshold levels". Arthritis Rheum. 38 (7): 926–38. doi:10.1002/art.1780380708. PMID 7612042.
- ↑ Kwiatek R, Barnden L, Tedman R, Jarrett R, Chew J, Rowe C, Pile K (December 2000). "Regional cerebral blood flow in fibromyalgia: single-photon-emission computed tomography evidence of reduction in the pontine tegmentum and thalami". Arthritis Rheum. 43 (12): 2823–33. doi:10.1002/1529-0131(200012)43:12<2823::AID-ANR24>3.0.CO;2-E. PMID 11145042.
- ↑ Gracely RH, Petzke F, Wolf JM, Clauw DJ (May 2002). "Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia". Arthritis Rheum. 46 (5): 1333–43. doi:10.1002/art.10225. PMID 12115241.
- ↑ Cook DB, Lange G, Ciccone DS, Liu WC, Steffener J, Natelson BH (February 2004). "Functional imaging of pain in patients with primary fibromyalgia". J. Rheumatol. 31 (2): 364–78. doi:10.1093/rheumatology/31.6.364. PMID 14760810.
- ↑ Burckhardt CS, Clark SR, Bennett RM (May 1991). "The fibromyalgia impact questionnaire: development and validation". J. Rheumatol. 18 (5): 728–33. PMID 1865419.
- ↑ Harris RE, Sundgren PC, Pang Y, Hsu M, Petrou M, Kim SH, McLean SA, Gracely RH, Clauw DJ (March 2008). "Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in fibromyalgia". Arthritis Rheum. 58 (3): 903–7. doi:10.1002/art.23223. PMID 18311814.
- ↑ Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC (April 2007). "Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain?". J Neurosci. 27 (15): 4004–7. doi:10.1523/JNEUROSCI.0098-07.2007. PMID 17428976.
- ↑ Wood PB, Patterson JC, Sunderland JJ, Tainter KH, Glabus MF, Lilien DL (January 2007). "Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: a pilot study". J Pain. 8 (1): 51–8. doi:10.1016/j.jpain.2006.05.014. PMID 17023218.
- ↑ Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, Bushnell MC, Chizh BA (June 2007). "Fibromyalgia patients show an abnormal dopamine response to pain". Eur J Neurosci. 25 (12): 3576–82. doi:10.1111/j.1460-9568.2007.05623.x. PMID 17610577.
- ↑ Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK (September 2007). "Decreased central mu-opioid receptor availability in fibromyalgia". J Neurosci. 27 (37): 10000–6. doi:10.1523/JNEUROSCI.2849-07.2007. PMID 17855614.
- ↑ Dehghan, Mahboobeh; Schmidt-Wilcke, Tobias; Pfleiderer, Bettina; Eickhoff, Simon B.; Petzke, Frank; Harris, Richard E.; Montoya, Pedro; Burgmer, Markus (1 May 2016). "Coordinate-based (ALE) meta-analysis of brain activation in patients with fibromyalgia". Human Brain Mapping. 37 (5): 1749–1758. doi:10.1002/hbm.23132. ISSN 1097-0193.
- ↑ http://www.niams.nih.gov/Health_Info/Fibromyalgia/default.asp
- ↑ http://www.rheumatology.org/Practice/Clinical/Patients/Diseases_And_Conditions/Fibromyalgia/
- ↑ Wolfe F (August 2003). "Stop using the American College of Rheumatology criteria in the clinic". J. Rheumatol. 30 (8): 1671–2. PMID 12913920.
- 1 2 3 4 Wolfe, F; et al. (May 2010). "The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity" (PDF). Arthritis Care Res. 62 (5): 600–610. doi:10.1002/acr.20140. PMID 20461783.
- ↑ Nam TS, Choi SY, Park DJ, Lee SS, Kim YO, Kim MK (2015). "The Overlap between Fibromyalgia Syndrome and Myotonia Congenita". J Clin Neurol. 11 (2): 188–91. doi:10.3988/jcn.2015.11.2.188.
- ↑ Colding-Jørgensen E (2005). "Phenotypic variability in myotonia congenita". Muscle Nerve. 32 (1): 19–34. doi:10.1002/mus.20295. PMID 15786415.
- ↑ Lossin C, George AL Jr (2008). "Myotonia congenita". Adv Genet. 63: 25–55. doi:10.1016/S0065-2660(08)01002-X. PMID 19185184.
- 1 2 Goldenberg DL (2008). "Multidisciplinary modalities in the treatment of fibromyalgia". J Clin Psychiatry. 69 (2): 30–4. PMID 18537461.
- 1 2 Sommer C, Häuser W, Alten R, Petzke F, Späth M, Tölle T, Uçeyler N, Winkelmann A, Winter E, Bär KJ (June 2012). "Drug therapy of fibromyalgia syndrome. Systematic review, meta-analysis and guideline" (PDF). Schmerz. 26 (3): 297–310. doi:10.1007/s00482-012-1172-2. PMID 22760463.
- 1 2 Carville SF, Arendt-Nielsen S, Bliddal H, Blotman F, Branco JC, Buskila D, Da Silva JA, Danneskiold-Samsøe B, Dincer F, Henriksson C, Henriksson KG, Kosek E, Longley K, McCarthy GM, Perrot S, Puszczewicz M, Sarzi-Puttini P, Silman A, Späth M, Choy EH (April 2008). "EULAR evidence-based recommendations for the management of fibromyalgia syndrome" (PDF). Ann. Rheum. Dis. 67 (4): 536–41. doi:10.1136/ard.2007.071522. PMID 17644548.
- 1 2 3 2012 Canadian guidelines for the diagnosis and management of fibromyalgia syndrome
- ↑ "FDA Approves First Drug for Treating Fibromyalgia" (Press release). U.S. Food and Drug Administration. 21 June 2007. Retrieved 14 January 2008.
- ↑ European Medicines Agency. "Questions and answers on the recommendati on for the refusal of the marketing authorisation for Milnacipran Pierre Fabre Médicament/Impulsor" (PDF). European Medicines Agency. Retrieved 30 May 2013.
- ↑ Häuser W, Bernardy K, Uçeyler N, Sommer C (January 2009). "Treatment of fibromyalgia syndrome with antidepressants: a meta-analysis". JAMA. 301 (2): 198–209. doi:10.1001/jama.2008.944. PMID 19141768.
- ↑ Häuser W, Wolfe F, Tölle T, Uçeyler N, Sommer C (April 2012). "The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis". CNS Drugs. 26 (4): 297–307. doi:10.2165/11598970-000000000-00000. PMID 22452526.
- ↑ Derry S, Gill D, Phillips T, Moore RA (14 March 2012). Derry, Sheena, ed. "Milnacipran for neuropathic pain and fibromyalgia in adults". Cochrane database of systematic reviews (Online). 3: CD008244. doi:10.1002/14651858.CD008244.pub2. PMID 22419330.
- ↑ Moore, RA; Derry, S; Aldington, D; Cole, P; Wiffen, PJ (31 July 2015). "Amitriptyline for fibromyalgia in adults.". The Cochrane database of systematic reviews. 7: CD011824. doi:10.1002/14651858.CD011824. PMID 26230384.
- ↑ Häuser, Winfried; Walitt, Brian; Fitzcharles, Mary-Ann; Sommer, Claudia (1 January 2014). "Review of pharmacological therapies in fibromyalgia syndrome". Arthritis Research & Therapy. 16: 201. doi:10.1186/ar4441. ISSN 1478-6362.
- 1 2 Moore RA, Wiffen PJ, Derry S, McQuay HJ (16 March 2011). Moore, R Andrew, ed. "Gabapentin for chronic neuropathic pain and fibromyalgia in adults". Cochrane database of systematic reviews (Online) (3): CD007938. doi:10.1002/14651858.CD007938.pub2. PMID 21412914.
- ↑ Üçeyler N, Sommer C, Walitt B, Häuser W (2013). "Anticonvulsants for fibromyalgia". Cochrane Database Syst Rev. 10: CD010782. doi:10.1002/14651858.CD010782. PMID 24129853.
- ↑ Derry, Sheena; Cording, Malene; Wiffen, Philip J.; Law, Simon; Phillips, Tudor; Moore, R. Andrew (2016-09-29). "Pregabalin for pain in fibromyalgia in adults". The Cochrane Database of Systematic Reviews. 9: CD011790. doi:10.1002/14651858.CD011790.pub2. ISSN 1469-493X. PMID 27684492.
- ↑ Straube S, Moore RA, Paine J, Derry S, Phillips CJ, Hallier E, McQuay HJ (2011). "Interference with work in fibromyalgia – effect of treatment with pregabalin and relation to pain response". BMC Musculoskelet Disord. 12: 125. doi:10.1186/1471-2474-12-125. PMC 3118156
 . PMID 21639874.
. PMID 21639874. - 1 2 MacLean, AJ; Schwartz, TL (May 2015). "Tramadol for the treatment of fibromyalgia.". Expert Review of Neurotherapeutics. 15 (5): 469–75. doi:10.1586/14737175.2015.1034693. PMID 25896486.
- ↑ Schug SA. Combination analgesia in 2005 – a rational approach: focus on paracetamol-tramadol. Clinical rheumatology. 2006;25(Supplement). doi:10.1007/s10067-006-0202-9. PMID 16741784.
- ↑ Ngian GS, Guymer EK, Littlejohn GO (February 2011). "The use of opioids in fibromyalgia". Int J Rheum Dis. 14 (1): 6–11. doi:10.1111/j.1756-185X.2010.01567.x. PMID 21303476.
- ↑ Berger A. Patterns of use of opioids in patients with fibromyalgia In: EULAR; 2009:SAT0461
- ↑ Jones KD, Deodhar P, Lorentzen A, Bennett RM, Deodhar AA (2007). "Growth hormone perturbations in fibromyalgia: a review". Seminars in Arthritis and Rheumatism. 36 (6): 357–79. doi:10.1016/j.semarthrit.2006.09.006. PMID 17224178.
- ↑ Cuatrecasas, G.; Alegre, C.; Casanueva, FF. (June 2014). "GH/IGF1 axis disturbances in the fibromyalgia syndrome: is there a rationale for GH treatment?". Pituitary. 17 (3): 277–83. doi:10.1007/s11102-013-0486-0. PMID 23568565.
- ↑ Staud R (August 2011). "Sodium oxybate for the treatment of fibromyalgia". Expert Opin Pharmacother. 12 (11): 1789–98. doi:10.1517/14656566.2011.589836. PMID 21679091.
- ↑ See S, Ginzburg R. (1 August 2008). "Choosing a Skeletal Muscle Relaxant". Am Fam Physician. 78 (3): 365–70.
- ↑ Heymann RE, Paiva Edos S, Helfenstein M, Pollak DF, Martinez JE, Provenza JR, Paula AP, Althoff AC, Souza EJ, Neubarth F, Lage LV, Rezende MC, de Assis MR, Lopes ML, Jennings F, Araújo RL, Cristo VV, Costa ED, Kaziyama HH, Yeng LT, Iamamura M, Saron TR, Nascimento OJ, Kimura LK, Leite VM, Oliveira J, de Araújo GT, Fonseca MC (2010). "Brazilian consensus on the treatment of fibromyalgia". Rev Bras Reumatol. 50 (1): 56–66. doi:10.1590/S0482-50042010000100006. PMID 21125141.
- ↑ Holman AJ (September 2009). "Impulse control disorder behaviors associated with pramipexole used to treat fibromyalgia". J Gambl Stud. 25 (3): 425–31. doi:10.1007/s10899-009-9123-2. PMID 19241148.
- ↑ Späth, M (May 2002). "Current experience with 5-HT3 receptor antagonists in fibromyalgia.". Rheumatic diseases clinics of North America. 28 (2): 319–28. PMID 12122920.
- ↑ Younger J, Parkitny L, McLain D (2014). "The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain". Clin Rheumatol. 33 (4): 451–459. doi:10.1007/s10067-014-2517-2. PMC 3962576
 . PMID 24526250.
. PMID 24526250. - ↑ Arnold, L.M.; Gebke, K.B.; Choy, E.H.S. (2016). "Fibromyalgia: Management strategies for primary care providers". The International Journal of Clinical Practice. 70: 99–112. doi:10.1111/ijcp.12757.
- ↑ Clauw, D. (2014). "Fibromyalgia: A clinical review". Journal of the American Medical Association. 311: 1547–1555. doi:10.1001/jama.2014.3266.
- ↑ Okifuji, A.; Hare, B.D. (2013). "Management of fibromyalgia syndrome: Review of evidence". Pain Therapy. 2: 87–104. doi:10.1007/s40122-013-0016-9.
- ↑ Williams, A.C.D.; Eccleston, C.; Morley, S. (2009). "Psychological therapies for the management of chronic pain (excluding headache) in adults.". Cochraine Database Systematic Review. 11: 108–111. doi:10.1002/14651858.CD007407.pub3.
- ↑ Abeles, M.; Solitar, B.M.; Pillinger, M.H.; Abeles, A.M. (2008). "Update of fibromyalgia therapy". American Journal of Medicine. 121: 555–561. doi:10.1016/j.amjmed.2008.02.036.
- ↑ Hauser W, Bernardy K, Arnold B, Offenbacher M, Schiltenwolf M (2009). "Efficacy of multicomponent treatment in fibromyalgia syndrome: A meta-analysis of randomized controlled clinical trials". Arthritis Care & Research. 61 (2): 216–224. doi:10.1002/art.24276.
- ↑ Arnold, L.M.; Clauw, D.; Dunegan, J.; Turk, D.C. (2012). "A framework for fibromyalgia management for primary care providers". Mayo Clinic Proceedings. 87: 488–496. doi:10.1016/j.mayocp.2012.02.010.
- ↑ Glombiewski, J.A.; Sawyer, A.T.; Gutermann, A.T.; Koenig, K.; Rief, W.; Hofmann, S.G. (2010). "Psychological treatments for fibromyalgia: A meta-analysis". Pain. 151: 280–295. doi:10.1016/j.pain.2010.06.011.
- ↑ Okifuji, A.; Hare, B.C. (2013). "Management of fibromyalgia syndrome: Review of evidence". Pain Therapy. 2: 87–104. doi:10.1007/s40122-013-0016-9.
- ↑ Howard, S.; Smith, M.D.; Barkin, R.L. (2011). "Fibromyalgia syndrome: a discussionof the syndrome and pharmacotherapy". American Journal of Therapeutics. 17: 418–439. doi:10.1097/MJT.0b013e3181df8e1b.
- ↑ Bernardy K, Klose P, Busch AJ, Choy EH, Häuser W (10 September 2013). "Cognitive behavioral therapies for fibromyalgia.". The Cochrane database of systematic reviews. 9: CD009796. doi:10.1002/14651858.CD009796.pub2. PMID 24018611.
- ↑ Glombiewski JA, Sawyer AT, Gutermann J, Koenig K, Rief W, Hofmann SG (November 2010). "Psychological treatments for fibromyalgia: a meta-analysis". Pain. 151 (2): 280–95. doi:10.1016/j.pain.2010.06.011. PMID 20727679.
- ↑ Glombiewski, J.A.; Sawyer, A.T.; Gutermann, A.T.; Koenig, K.; Rief, W.; Hofmann, S.G. (2010). "Psychological treatments for fibromyalgia: A meta analysis". Pain. 151: 280–295. doi:10.1016/j.pain.2010.06.011.
- ↑ Williams DA (August 2003). "Psychological and behavioral therapies in fibromyalgia and related syndromes". Best Pract Res Clin Rheumatol. 17 (4): 649–65. doi:10.1016/S1521-6942(03)00034-2. PMID 12849717.
- ↑ Bernardy K, Füber N, Köllner V, Häuser W (October 2010). "Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome – a systematic review and meta-analysis of randomized controlled trials". J. Rheumatol. 37 (10): 1991–2005. doi:10.3899/jrheum.100104. PMID 20682676.
- ↑ Busch A, Schachter CL, Peloso PM, Bombardier C (2002). Busch, Angela, ed. "Exercise for treating fibromyalgia syndrome". Cochrane database of systematic reviews (Online) (3): CD003786. doi:10.1002/14651858.CD003786. PMID 12137713.
- ↑ Gowans SE, deHueck A (2004). "Effectiveness of exercise in management of fibromyalgia". Current Opinion in Rheumatology. 16 (2): 138–42. doi:10.1097/00002281-200403000-00012. PMID 14770100.
- 1 2 Goldenberg DL, Burckhardt C, Crofford L (Nov 2004). "Management of fibromyalgia syndrome" (Free full text). Journal of the American Medical Association. 292 (19): 2388–2395. doi:10.1001/jama.292.19.2388. ISSN 0098-7484. PMID 15547167.
- ↑ Mannerkorpi K, Nyberg B, Ahlmén M, Ekdahl C (2000). "Pool exercise combined with an education program for patients with fibromyalgia syndrome: a prospective, randomized study". Journal of Rheumatology. 27 (10): 2473–2481. PMID 11036846.
- ↑ http://www.chop.edu/service/amplified-musculoskeletal-pain-syndrome/about-amps/amps-treatment.html
- ↑ Goldenberg DL, Mossey CJ, Schmid CH (December 1995). "A model to assess severity and impact of fibromyalgia". J. Rheumatol. 22 (12): 2313–8. PMID 8835568.
- ↑ Chakrabarty S, Zoorob R (July 2007). "Fibromyalgia". American Family Physician. 76 (2): 247–254. PMID 17695569. Retrieved 6 January 2008.
- ↑ Bartels EM, Dreyer L, Jacobsen S, Jespersen A, Bliddal H, Danneskiold-Samsøe B (2009). "Fibromyalgia, diagnosis and prevalence. Are gender differences explainable?". Ugeskrift for Lægerer. 171 (49): 3588–92. PMID 19954696.
- ↑ Health Information Team (February 2004). "Fibromyalgia". BUPA insurance.
- ↑ Yunus M, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL (Aug 1981). "Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched normal controls". Seminars in arthritis and rheumatism. 11 (1): 151–171. doi:10.1016/0049-0172(81)90096-2. ISSN 0049-0172. PMID 6944796.
- ↑ "Fibro-". Dictionary.com. Retrieved 21 May 2008.
- ↑ "Meaning of myo". Web.archive.org. 12 April 2009. Archived from the original on 12 April 2009. Retrieved 26 August 2012.
- ↑ "Meaning of algos". Web.archive.org. 12 April 2009. Archived from the original on 12 April 2009. Retrieved 26 August 2012.
- 1 2 Wolfe F (2009). "Fibromyalgia wars". J. Rheumatol. 36 (4): 671–8. doi:10.3899/jrheum.081180. PMID 19342721.
- ↑ Smythe HA, Moldofsky H (1977). "Two contributions to understanding of the "fibrositis" syndrome". Bull Rheum Dis. 28 (1): 928–31. PMID 199304.
- ↑ Winfield JB (June 2007). "Fibromyalgia and related central sensitivity syndromes: twenty-five years of progress". Semin. Arthritis Rheum. 36 (6): 335–8. doi:10.1016/j.semarthrit.2006.12.001. PMID 17303220. Retrieved 21 May 2008.
- 1 2 Inanici F, Yunus MB (October 2004). "History of fibromyalgia: past to present". Curr Pain Headache Rep. 8 (5): 369–78. doi:10.1007/s11916-996-0010-6. PMID 15361321.
- ↑ Goldenberg DL (May 1987). "Fibromyalgia syndrome. An emerging but controversial condition". JAMA. 257 (20): 2782–7. doi:10.1001/jama.257.20.2782. PMID 3553636.
- ↑ Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P (February 1990). "The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee". Arthritis Rheum. 33 (2): 160–72. doi:10.1002/art.1780330203. PMID 2306288.
- ↑ "High health care utilization and costs in patients with fibromyalgia". Drug Benefit Trends. 22 (4): 111. 2010.
- ↑ Berenson. "Drug Approved. Is Disease Real?". The New York Times. Retrieved 26 March 2014.
- ↑ http://www.news-medical.net/news/20130322/Fibromyalgia-an-interview-with-Dr-Frederick-Wolfe-University-of-Kansas-School-of-Medicine.aspx
- ↑ Goldenberg DL (January 1995). "Fibromyalgia: why such controversy?". Ann. Rheum. Dis. 54 (1): 3–5. doi:10.1136/ard.54.1.3. PMC 1005499
 . PMID 7880118.
. PMID 7880118. - ↑ Celeste Cooper; Jeffrey Miller (2010). Integrative Therapies for Fibromyalgia, Chronic Fatigue Syndrome, and Myofascial Pain: The Mind-Body Connection. Inner Traditions / Bear & Co. p. 114. ISBN 1594773238. Retrieved 16 July 2012.
- ↑ Kroenke K, Harris L (May 2001). "Symptoms research: a fertile field". Annals of Internal Medicine. 134 (9 Pt 2): 801–802. doi:10.7326/0003-4819-134-9_Part_2-200105011-00001. ISSN 0003-4819. PMID 11346313.
- ↑ Kanaan RA, Lepine JP, Wessely SC (December 2007). "The association or otherwise of the functional somatic syndromes". Psychosom Med. 69 (9): 855–9. doi:10.1097/PSY.0b013e31815b001a. PMC 2575798
 . PMID 18040094.
. PMID 18040094. - ↑ Wood PB, Holman AJ, Jones KD (June 2007). "Novel pharmacotherapy for fibromyalgia". Expert Opin Investig Drugs. 16 (6): 829–41. doi:10.1517/13543784.16.6.829. PMID 17501695.
- ↑ Aziz, I; Hadjivassiliou, M; Sanders, DS (September 2015). "The spectrum of noncoeliac gluten sensitivity.". Nature reviews. Gastroenterology & hepatology. 12 (9): 516–26. doi:10.1038/nrgastro.2015.107. PMID 26122473.
- ↑ Bennett RM, De Garmo P, Clark SR (1996). "A Randomized, Prospective, 12 Month Study To Compare The Efficacy Of Guaifenesin Versus Placebo In The Management Of Fibromyalgia" (reprint). Arthritis and Rheumatism. 39 (10): S212. doi:10.1002/art.1780391004.
- ↑ Kristin Thorson (1997). "Is One Placebo Better Than Another? – The Guaifenesin Story (Lay summary and report)". Fibromyalgia Network. Fibromyalgia Network.
External links
| Wikimedia Commons has media related to Fibromyalgia. |
- Fibromyalgia at DMOZ
- Arthritis – Types – Fibromyalgia by the CDC
- Questions and Answers About Fibromyalgia by the National Institute of Arthritis and Musculoskeletal and Skin Diseases
- National Institute of Arthritis and Musculoskeletal and Skin Diseases - US National Institute of Arthritis and Musculoskeletal and Skin Diseases