Female cosmetic coalitions

The theory of Female Cosmetic Coalitions (FCC) is a new and controversial attempt to explain the evolutionary emergence of art, ritual and symbolic culture in Homo sapiens. It is proposed by evolutionary anthropologists Chris Knight and Camilla Power together with archaeologist Ian Watts.[1][2][3][4]
Supporters of this new theory contest the prevailing assumption that the earliest art was painted or engraved on external surfaces such as cave walls or rock faces. They argue instead that art is much older than previously thought and that the canvas was initially the human body. The earliest art, according to FCC, consisted of predominantly blood-red designs produced on the body for purposes of cosmetic display.[5]
'Female Cosmetic Coalitions' is a conceptual approach linking (a) Darwin's theory of evolution by natural and sexual selection, (b) research into sexual signalling by wild-living monkeys and apes, (c) the fossil record of encephalization in human evolution, (d) recent archaeological discoveries of red ochre pigments dating back to the speciation in Africa of Homo sapiens around 250,000 years ago and (e) modern hunter-gatherer ethnography.[6][7][8][9] These seemingly divergent topics are brought together in a co-authored publication attempting to explain why the world today is populated by modern Homo sapiens instead of by the equally large-brained, previously successful Neanderthals.[10] An account of exhaustive archaeological testing of the FCC theory, including robust debate between specialists, is this article published in the journal Current Anthropology in 2016.[11]
‘Of course, not everyone is convinced, but anthropologists are starting to take the idea seriously. One of its strengths is that it addresses the question of why symbolic culture evolved, rather than simply how it did so, according to Robin Dunbar from the University of Liverpool.’
Details of the FCC model
Reproductive synchrony
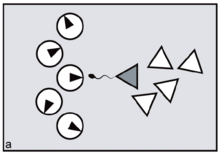

In primates, reproductive synchrony usually takes the form of conception and birth seasonality.[12] The regulatory 'clock', in this case, is the sun's position in relation to the tilt of the earth. In nocturnal or partly nocturnal primates — for example, owl monkeys — the periodicity of the moon may also come into play.[13][14] Synchrony in general is for primates an important variable determining the extent of 'paternity skew' — defined as the extent to which fertile matings can be monopolised by a fraction of the population of males. The greater the precision of female reproductive synchrony — the greater the number of ovulating females who must be guarded simultaneously — the harder it is for any dominant male to succeed in monopolising a harem all to himself. This is simply because, by attending to any one fertile female, the male unavoidably leaves the others at liberty to mate with his rivals. The outcome is to distribute paternity more widely across the total male population, reducing paternity skew (figures a, b).[15]
Concealed ovulation, synchrony and evolution



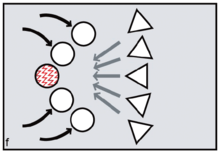

Reproductive synchrony can never be perfect. On the other hand, theoretical models predict that group-living species will tend to synchronise wherever females can benefit by maximising the number of males offered chances of paternity, minimising reproductive skew.[16] The same models predict that female primates, including evolving humans, will tend to synchronise wherever fitness benefits can be gained by securing access to multiple males. Conversely, group-living females who need to restrict paternity to a single dominant harem-holder should assist him by avoiding synchrony.[17][18]
In the human case, according to FCC, evolving females with increasingly heavy childcare burdens would have done best by resisting attempts at harem-holding by locally dominant males. No human female needs a partner who will get her pregnant only to disappear, abandoning her in favour of his next sexual partner.[19] To any local group of females, the more such philandering can be successfully resisted — and the greater the proportion of previously excluded males who can be included in the breeding system and persuaded to invest effort — the better.[20] By evolving concealed ovulation and continuous receptivity, females force males into longer periods of consortship if they are to have a good chance of achieving impregnation (figures c,d).[21] Reproductive synchrony — whether seasonal, lunar or a combination of the two — is a key strategy for reproductive levelling, reducing paternity skew and involving more males in investment in offspring.[22] Greater reproductive synchrony owing to seasonality during glacial cycles may have differentiated Neanderthal reproductive strategies from those of Homo sapiens ancestors.[23]
Costs of increasing brain-size
In this model, the factor driving female strategies is the high cost to females of increasingly large-brained offspring, requiring increased investment from males. As females of Homo heidelbergensis, the ancestor of Neanderthals and modern humans, came under increasing selection pressure for larger brain size within the past half million years,[24] they needed more energy in support. This meant greater productivity by males as hunters. However, in the Darwinian world of primate sexual competition, males may be more interested in finding new fertile females than in supplying the needs of breast-feeding mothers and their infants. Women, unlike chimpanzees, do not show their fertile time. But females cannot easily disguise menstruation. Menstrual periods mark out very clearly which females are coming close to fertility among other females who are pregnant and lactating. Dominant males could therefore target the cycling females and neglect those most in need of support.
Menstruation, synchrony and cosmetics
FCC proponents argue that menstruation became a key problem because it potentially creates conflicts between the females and conflicts between males. Menstruation has little social impact among chimpanzees or bonobos, since visible oestrus swellings are the focus of male attention. But once external signs of ovulation had been phased out in the human lineage, according to FCC, menstruation became salient as the one remaining external promise of fertility. Potentially, dominant males might exploit such information, repeatedly targeting newly cycling females at the expense of pregnant or nursing mothers (figure e). Those who might lose male investment needed to take control. According to FCC, older and more experienced females did this by initiating newly cycling females into their kin-based coalitions (figure f, g). Red ochre pigments allowed women to take conscious control over their signals, resisting any dominant male strategy of picking and choosing between them on biological grounds.[25][26] It is argued that because precise, sustained menstrual synchrony is difficult to achieve, painting up with blood-red pigments was the next best thing, enabling the benefits of artificial, ritually constructed synchrony.[27]
How does this affect male strategies?
To explore a male strategic point of view, proponents of FCC make a simple model of alternative strategies.[28] Female A uses cosmetics as part of her ritual coalition whenever one of them menstruates; Female B and all her female neighbors use no cosmetics. Male A is prepared to work/invest to gain access; Male B tries a philanderer strategy, moving to the next cycling fertile female, neglecting the previous partner once she is pregnant. Very quickly, Male A will end up working/doing bride-service for Female A’s coalition, since he has no competition from Male B. Male A gains regular fitness as a result. Male B will pair up with Female B, but is then liable to abandon her if he finds a new cycling female. She then has little support during pregnancy/breastfeeding. The question will be whether Male B gains sufficient fitness via a roving strategy of picking up cycling, non-cosmetic females. If Male A is not able to compete with Male B in terms of dominance, he is better off choosing the cosmetic females. Because Female B and her non-cosmetic female neighbors get the attentions, but no reliable investment, from Male B, they discourage any investment from the likes of Male A. Once costs of encephalization begin to bite and cooperative strategies are needed to support offspring, how many females will be choosing philanderers in preference to investors? Those females are not likely to be ancestors of large-brained hominins like ourselves or the Neanderthals.
Reverse dominance, sacredness and taboo
Female strategies of counter-dominance culminated, according to this body of theory, in the eventual overthrow of primate-style dominance and its replacement by hunter-gatherer-style 'reverse dominance'. 'Reverse dominance' is defined by evolutionary anthropologists as an inverted social hierarchy — rule from below by an ungovernable community. To maintain an egalitarian social order, individuals band together to resist being dominated by anyone.[31]
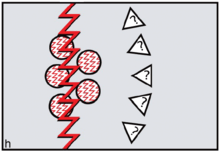
The FCC model predicts the specific form of reverse dominance display needed by female coalitions resisting would-be dominant or philanderer males. Females needed to signal 'No' by constructing themselves as inviolable using red cosmetics. To assert ritual power, they needed to go periodically on 'sex strike'.[32] To defend themselves physically against harassment by non-kin males, defiant females needed to draw on the support of male kin — sons and brothers — as members of their reverse dominance coalitions. In order to reverse signals of sexual availability, it was logical to sing and dance an unmistakable message: 'Wrong species, wrong sex, wrong time!'. On this basis, FCC theory leads us to expect 'divine' or 'totemic' spiritual entities depicted in early rock art to be therianthropic ('wrong species'), gender-ambivalent ('wrong sex') and blood-red ('wrong time') (figure h).[33][34]
This brings FCC into line with Ḗmile Durkheim, who argued that the earliest divine beings were ritually generated representations of society.[35] Durkheim's 'society', according to FCC, was in the first instance the bottom-up authority of Female Cosmetic Coalitions.
Interpreting the ochre record
"The use of ocher is particularly intensive: it is not unusual to find a layer of the cave floor impregnated with a purplish red to a depth of eight inches. The size of these ocher deposits raises a problem not yet solved. The colouring is so intense that practically all the loose ground seems to consist of ocher. One can imagine that the Aurignacians regularly painted their bodies red, dyed their animal skins, coated their weapons, and sprinkled the ground of their dwellings, and that a paste of ocher was used for decorative purposes in every phase of their domestic life. We must assume no less, if we are to account for the veritable mines of ocher on which some of them lived..."— Leroi-Gourhan, A. 1968. The Art of Prehistoric Man in Western Europe. London: Thames & Hudson, p. 40.
It was once thought that art and symbolic culture first emerged in Europe some 40,000 years ago, during the Middle-to-Upper Palaeolithic transition – often termed the 'symbolic explosion' or 'Upper Palaeolithic revolution'. Some archaeologists still adhere to this view. Others now accept that symbolic culture probably emerged in sub-Saharan Africa at a much earlier date, during the period known as the Middle Stone Age.[36] The evidence consists of traditions of ground ochre with strong selection for the colour red, examples of so-called ochre 'crayons' which appear to have been used for purposes of design, probably on the body, and geometric engravings on blocks of ochre. All this apparently formed part of a cosmetics industry dated to between 100,000 and 200,000 years ago.[37][38] In addition, from about 100,000 years ago, we have pierced shells which appear to show signs of wear, suggesting that they were strung together to make necklaces. If the ochre tradition has been correctly interpreted, it constitutes evidence for the world's first 'art' — an aspect of 'symbolic culture' — in the form of personal ornamentation and body-painting.[39][40] An alternative viewpoint is that pigment-only decorative systems are merely individualistic display, not necessarily indicative of ritual, whereas the bead traditions testify to language, institutionalised relationships and full-scale ritual and symbolic culture.[41][42]
Implications for the origins of language
Proponents of this model claim that it helps to explain when and how language in our species emerged. Among 'Machiavellian', competitive nonhuman primates, sex is a major source of conflict, mutual suspicion and mistrust, as a result of which group members attempt to minimise the cost of deception by responding only to bodily signals which are intrinsically 'hard to fake'. This social pressure from receivers prevents language from even beginning to emerge. FCC theorists argue that for signals as cheap and intrinsically unreliable as words to become socially accepted, unprecedentedly intense levels of in-group trust were required. An effect of the Female Cosmetic Coalitions strategy, claim its supporters, was to minimise internal sexual conflict within each gender group, giving rise to a trusting social atmosphere such as is found among extant human egalitarian hunter-gatherers. These new levels of public trust, according to supporters of the model, enabled our species' latent linguistic capacities to flourish where previously they had been suppressed.[43]
'Too many candidate theories are either too vague, or make predictions that fall outside the available evidence. In contrast, a good example in this regard is the Female Cosmetic Coalitions Model, which does provide specific testable predictions.'— Johansson, S. 2014. How can a social theory of language evolution be grounded in evidence? In D. Dor, C. Knight and J. Lewis (eds), The Social Origins of Language. Oxford: Oxford University Press, pp. 64-56
Testable predictions of the model
Its supporters claim that FCC is the only Darwinian theory to explain why there is so much red ochre in the early archaeological record of modern humans and why modern humans are then associated with red ochre wherever they went as they emerged from Africa. It is claimed that, more than any other theoretical model of modern human origins, FCC offers detailed and specific predictions testable in the light of data from a wide variety of disciplines.[44]
Archaeology
• Earliest evidence of symbolic behaviour should be found in a cosmetics industry focused on blood-red pigments.
Palaeontology
• The time-window should fit with fossil evidence for encephalization rates. On this basis, the earliest onset of the strategy should neither pre-date c. 600,000 B.P. nor post-date c. 150,000 B.P., by which time modern levels of cranial capacity had evolved.
Kinship and male investment
• Matrilocal residence with bride-service is expected as the initial situation.
Ethnography of magico-religious symbolism
• Counter-dominance should generate collective counter-reality. The first gods are therefore expected to be represented as WRONG + RED (wrong species/sex/time).
• Hunters' prohibitions on sex and menstruation are expected to operate within a lunar/menstrual cosmology.
Results
Proponents of FCC argue that these and other predictions should be easy, in principle, to falsify. The theory is currently being debated,[45] having received significant media coverage.[46] Despite this, not all scholars agree and the model remains controversial.[47][48]
See also
References
- ↑ Power, C. 2009. Sexual selection models for the emergence of symbolic communication: why they should be reversed. In R. Botha and C. Knight (eds), The Cradle of Language. Oxford: Oxford University Press, pp. 257-280.
- ↑ Power, C. 2010. Cosmetics, identity and consciousness. Journal of Consciousness Studies 17, No. 7-8, pp. 73-94
- ↑ Knight, C. 1991. Blood Relations. Menstruation and the origins of culture. New Haven and London: Yale University Press.
- ↑ Watts, I. M. Chazan and J. Wilkins, 2016. Early Evidence for Brilliant Ritualized Display: Specularite Use in the Northern Cape (South Africa) between ~500 and ~300 Ka. Current Anthropology Volume 57, Number 3, pp. 287-310.
- ↑ Watts, I. 2002. Ochre in the Middle Stone Age of southern Africa: Ritualised display or hide preservative? South African Archaeological Bulletin 57: 15-30.
- ↑ Power, C. 2004. Women in prehistoric art. In G. Berghaus (ed.), New Perspectives in Prehistoric Art. Westport, CT & London: Praeger, pp. 75-104.
- ↑ Power, C. and L. C. Aiello 1997. Female proto-symbolic strategies. In L. D. Hager (ed.), Women in Human Evolution. New York and London: Routledge, pp. 153-171.
- ↑ Power, C. 1999. Beauty magic: the origins of art. In R. Dunbar, C. Knight and C. Power (eds), The Evolution of Culture. Edinburgh: Edinburgh University Press, pp. 92-112.
- ↑ Power, C. 2009. Sexual selection models for the emergence of symbolic communication: why they should be reversed. In R. Botha and C. Knight (eds), The Cradle of Language. Oxford: Oxford University Press, pp. 257-280.
- ↑ Power, C., V. Sommer and I. Watts, 2013. The Seasonality Thermostat: Female Reproductive Synchrony and Male Behavior in Monkeys, Neanderthals, and Modern Humans. PaleoAnthropology 2013: 33−60. doi:10.4207/PA.2013.ART79
- ↑ Watts, I. M. Chazan and J. Wilkins, 2016. Early Evidence for Brilliant Ritualized Display: Specularite Use in the Northern Cape (South Africa) between ~500 and ~300 Ka. Current Anthropology Volume 57, Number 3, pp. 287-310.
- ↑ Brockman, D. K. and C. P. Van Schaik, 2005. Seasonality and reproductive function. In D. K. Brockman and C. P. van Schaik (eds), Seasonality in Primates. Studies of living and extinct human and non-human primates. Cambridge: Cambridge University Press, pp. 269-305.
- ↑ Fernandez-Duque, H. de la Iglesa and H. G. Erkert, 2010. Moonstruck primates: Owl monkeys (Aotus) need moonlight for nocturnal activity in their natural environment. PLoS ONE 5(9): e12572. doi10.1371/journal.pone.0012572
- ↑ Nash, L. T. 2007. Moonlight and behavior in nocturnal and cathemeral primates, especially Lepilemur leucopus: Illuminating possible anti-predator efforts. In S.L. Gursky and K.A.I. Nekaris (eds), Primate Anti-Predator Strategies. New York: Springer, pp. 173-205.
- ↑ Ostner, J, C. L. Nunn and O. Schülke 2008. Female reproductive synchrony predicts skewed paternity across primates. Behavioral Ecology doi:10.1093/beheco/arn093
- ↑ Knowlton, N. 1979. Reproductive synchrony, parental investment and the evolutionary dynamics of sexual selection. Animal Behavior 27: 1022-33.
- ↑ Turke, P. W. 1984. Effects of ovulatory concealment and synchrony on protohominid mating systems and parental roles. Ethology and Sociobiology 5: 33-44.
- ↑ Turke, P. W. 1988. Concealed ovulation, menstrual synchrony and paternal investment. In E. Filsinger (ed.), Biosocial Perspectives on the Family. Newbury Park, CA: Sage, pp. 119-136.
- ↑ Power, C. and L. C. Aiello 1997. Female proto-symbolic strategies. In L. D. Hager (ed.), Women in Human Evolution. New York and London: Routledge, pp. 153-171.
- ↑ Bowles, S. 2006. Group competition, reproductive levelling, and the evolution of human altruism. Science 314: 1569-1572.
- ↑ Turke, P. W. 1988. Concealed ovulation, menstrual synchrony and paternal investment. In E. Filsinger (ed.), Biosocial Perspectives on the Family. Newbury Park, CA: Sage, pp. 119-136.
- ↑ Power, C. Power, C., C. Arthur and L. C. Aiello 1997. On seasonal reproductive synchrony as an evolutionarily stable strategy in human evolution. Current Anthropology 38(1): 88-91.
- ↑ Power, C.; Sommer, V.; Watts, I. (2013). "The Seasonality Thermostat: Female Reproductive Synchrony and Male Behavior in Monkeys, Neanderthals, and Modern Humans" (PDF). PaleoAnthropology: 33−60. doi:10.4207/PA.2013.ART79.
- ↑ De Miguel, C. and M. Henneberg. 2001. Variation in hominid brain size: how much is due to method? Homo 52: 3–58.
- ↑ Knight, C. 1996. Menstruation. In A. Barnard & J. Spencer (eds), Encyclopedia of Social and Cultural Anthropology. London & New York: Routledge, pp. 363-4.
- ↑ Power, C. 1999. Beauty magic: the origins of art. In R. Dunbar, C. Knight and C. Power (eds), The Evolution of Culture. Edinburgh: Edinburgh University Press, pp. 92-112.
- ↑ Power, C. and L. C. Aiello 1997. Female proto-symbolic strategies. In L. D. Hager (ed.), Women in Human Evolution. New York and London: Routledge, pp. 153-171.
- ↑ Power, C., V. Sommer and I. Watts, 2013. The Seasonality Thermostat: Female Reproductive Synchrony and Male Behavior in Monkeys, Neanderthals, and Modern Humans. PaleoAnthropology 2013: 33−60. doi:10.4207/PA.2013.ART79
- ↑ Lewis-Williams, J. D. 1981. Believing and Seeing. Symbolic meanings in Southern San rock paintings. London: Academic Press.
- ↑ Power, C., and Watts, I. (1999). ‘First Gender, Wrong Sex’, in H. L. Moore, T. Sanders, and B. Kaare (eds), Those who Play with Fire. Gender, fertility and transformation in East and Southern Africa. London and New Brunswick, NJ: Athlone Press, 101-132.
- ↑ Boehm, C. 1993. Egalitarian behaviour and reverse dominance hierarchy. Current Anthropology 34(3): 227-254.
- ↑ Knight, C. 1991. ‘The Sex Strike’. Chapter 4 in Blood Relations. Menstruation and the origins of culture. New Haven and London: Yale University Press, pp. 122-153. ISBN 0-300-04911-0
- ↑ Power, C. 2004. ‘Women in prehistoric art’. In G. Berghaus (ed.), New Perspectives in Prehistoric Art. Westport, CT & London: Praeger, pp. 75-104.
- ↑ Power, C. 2009. Sexual selection models for the emergence of symbolic communication: why they should be reversed. In R. Botha and C. Knight (eds), The Cradle of Language. Oxford: Oxford University Press, pp. 257-280.
- ↑ Durkheim, E. 1965 [1912]. The Elementary Forms of the Religious Life. New York: Free Press.
- ↑ Henshilwood, C. and C. W. Marean 2003. The origin of modern human behavior. Current Anthropology 44(5): 627-651.
- ↑ Knight, C.; Power, C.; Watts, I. (April 1995). "The Human Symbolic Revolution: A Darwinian Account" (PDF). Cambridge Archaeological Journal. 5 (1): 75–114. doi:10.1017/S0959774300001190.
- ↑ Watts, I. 2009. Red ochre, body painting, and language: interpreting the Blombos ochre. In R. Botha and C. Knight (eds), The Cradle of Language. Oxford: Oxford University Press, pp. 62-92.
- ↑ Power, C. 2010. Cosmetics, identity and consciousness. Journal of Consciousness Studies 17, No. 7-8, pp. 73-94.
- ↑ Watts, I. M. Chazan and J. Wilkins, 2016. Early Evidence for Brilliant Ritualized Display: Specularite Use in the Northern Cape (South Africa) between ~500 and ~300 Ka. Current Anthropology Volume 57, Number 3, pp. 287-310.
- ↑ Henshilwood, C. S. and B. Dubreuil 2009. Reading the artifacts: gleaning language skills from the Middle Stone Age in southern Africa. In R. Botha and C. Knight (eds), The Cradle of Language. Oxford: Oxford University Press, pp. 41-61.
- ↑ Kuhn, S. L. and Stiner, M. C. 2007. Body ornamentation as information technology: towards an understanding of the significance of beads. In P. Mellars, K. Boyle, O. Bar-Yosef and C. Stringer (eds), Rethinking the Human Revolution, Cambridge: McDonald Institute Research Monographs, pp. 45-54.
- ↑ Watts, I. 2009. Red ochre, body painting, and language: interpreting the Blombos ochre. In R. Botha and C. Knight (eds), The Cradle of Language. Oxford: Oxford University Press, pp. 62-92.
- ↑ Power, C., V. Sommer and I. Watts, 2013. The Seasonality Thermostat: Female Reproductive Synchrony and Male Behavior in Monkeys, Neanderthals, and Modern Humans. PaleoAnthropology 2013: 33−60. doi:10.4207/PA.2013.ART79
- ↑ Watts, I. M. Chazan and J. Wilkins, 2016. Early Evidence for Brilliant Ritualized Display: Specularite Use in the Northern Cape (South Africa) between ~500 and ~300 Ka. Current Anthropology Volume 57, Number 3, pp. 287-310.
- ↑ Kate Douglas, 2001. Painted Ladies. New Scientist, 13 October 2001.
- ↑ Stringer, C. 2011. The Origin of Our Species. London: Allen Lane, pp. 136-137.
- ↑ Watts, I. M. Chazan and J. Wilkins, 2016. Early Evidence for Brilliant Ritualized Display: Specularite Use in the Northern Cape (South Africa) between ~500 and ~300 Ka. Current Anthropology Volume 57, Number 3, pp. 287-310.
External links
- Douglas, K (13 October 2001). "Painted Ladies" (PDF). New Scientist.
- Knight, C. 1991. ‘The Sex Strike’. Chapter 4 in Blood Relations. Menstruation and the origins of culture. New Haven and London: Yale University Press, pp. 122–153. ISBN 0-300-04911-0
- Power, C.; Sommer, V.; Watts, I. (2013). "The Seasonality Thermostat: Female Reproductive Synchrony and Male Behavior in Monkeys, Neanderthals, and Modern Humans" (PDF). PaleoAnthropology: 33−60. doi:10.4207/PA.2013.ART79.
- [1]
- ↑ Watts, I. M. Chazan and J. Wilkins, 2016. Early Evidence for Brilliant Ritualized Display: Specularite Use in the Northern Cape (South Africa) between ~500 and ~300 Ka. Current Anthropology Volume 57, Number 3, pp. 287-310.