Nuclear organization
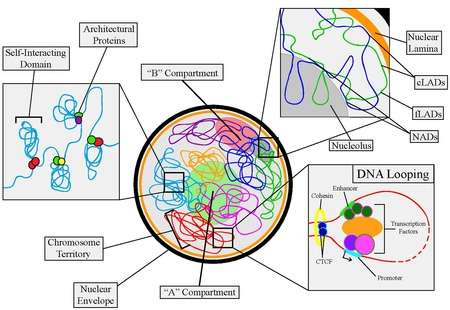
Nuclear organization refers to the spatial distribution of chromatin within a cell nucleus. There are many different levels and scales of nuclear organisation.
At the smallest scale, DNA is packaged into units called nucleosomes. The quantity and organisation of these nucleosomes can affect the accessibility of local chromatin. This has a knock-on effect on the expression of nearby genes, additionally determining whether or not they can be regulated by transcription factors.
At slightly larger scales, DNA looping can physically bring together DNA elements that would otherwise be separated by large distances. These interactions allow regulatory signals to cross over large genomic distances - for example, from enhancers to promoters.
In contrast, on a large-scale, the arrangement of chromosomes can determine their properties. Chromosomes are organised into two compartments labelled A ("active") and B ("inactive"), each with distinct properties. Moreover, entire chromosomes segregate into distinct regions called chromosome territories.
Importance
Cells within an organism have near identical nucleic acid sequences, but often exhibit different phenotypes. One way in which this individuality occurs is through changes in genome architecture, which can alter the expression of different sets of genes. These alterations can have a downstream effect on cellular functions such as cell cycle facilitation, DNA replication, nuclear transport, and alteration of nuclear structure. DNA packaging within the nucleus results in distinct configurations and regions to promote specific inter- and intra-chromatin, protein, and larger nuclear structure interactions. From DNA looping to formation of higher-order chromatin structures to chromosome territories, nuclear genome organization is essential for proper cellular function.
History and methodology
The organization of chromosomes into distinct regions within the nucleus was first proposed in 1885 by Carl Rabl. Later in 1909, with the help of the microscopy technology at the time, Theodor Boveri coined the termed chromosome territories after observing that chromosomes occupy individually distinct nuclear regions.[1] Since then, mapping genome architecture has become a major topic of interest.
Over the last ten years, rapid methodological developments have greatly advanced understanding in this field.[2] Large-scale DNA organization can be assessed with DNA imaging using fluorescent tags, such as DNA Fluorescence in situ hybridization (FISH), and specialized microscopes.[3] Additionally, high-throughput sequencing technologies such as Chromosome Conformation Capture-based methods can measure how often DNA regions are in close proximity.[4] At the same time, progress in genome-editing techniques (such as Crispr Cas9, ZFNs, and TALENs) have made it easier to assign organizational function to various architectural components, such as specific DNA regions and proteins.[5]
Architectural proteins
Architectural proteins regulate chromatin structure by establishing physical interactions between DNA elements.[6] These proteins tend to be highly conserved across a majority of eukaryotic species.
In mammals, key architectural proteins include:
- Histones: DNA is wrapped around histones to form nucleosomes, which are basic units of chromatin structure. Each nucleosome consists of 8 histone protein subunits, around which roughly 147 DNA base pairs are wrapped in 1.67 left-handed turns. Altogether, nucleosomes pack approximately 2 meters of double stranded DNA into a 10 µm diameter nucleus.[7] The concentration and specific composition of histones used can determine local chromatin structure. For example, euchromatin is a form of chromatin with low nucleosome concentration - here, the DNA is exposed, promoting interactions with gene expression, replication, and organizational machinery. In contrast, heterochromatin has high nucleosome concentration and is associated with repression of gene expression and replication, as the necessary proteins cannot interact with the DNA.
- Chromatin Remodeling Enzymes: These enzymes are responsible for promoting euchromatin or heterochromatin formation by a number of processes, particularly modifying histone tails or physically moving the nucleosomes. This in turn, helps regulate gene expression, replication, and how the chromatin interacts with architectural factors.[8] The list of chromatin remodeling enzymes is extensive and many have specific roles within the nucleus. For example, in 2016 Wiechens et al. recently identified two human enzymes, SNF2H and SNF2L, that are active in regulating CTCF binding and therefore affect genome organization and transcription of many genes.[9]
- CTCF: CCCTC-binding factor (CTCF), or 11-zinc finger protein, is considered to be the most prominent player in linking genome organization with gene expression. CTCF interacts with specific DNA sequences and a variety of other architectural proteins, chiefly cohesion (Rubio et al., 2008), to fact as transcriptional repressor, activator, and insulator by mediating DNA looping. As well, CTCF has been found to be a common factor for defining boundaries of self-interacting domains and anchoring the chromatin to the nuclear lamina (Guelen et al., 2008) both of which are the foundation of total genome organization. CTCF is also found to be involved in V(D)J recombination.[10]
- Cohesin: Initially discovered to be directly involved in binding sister chromatids to ensure proper segregation during mitosis, Cohesin has continually been linked to more functions within the cell.[11] It has been found to help facilitate DNA repair and recombination, meiotic chromosome pairing and orientation, chromosome condensation, DNA replication, gene expression, and genome architecture.[12] Cohesin is a heterodimer composed of the proteins SMC1 and SMC3 in combination with the SCC1 and SCC3 proteins. The entire complex is loaded onto DNA by the NIPBL-MAU2 complex in a ring like fashion.[13]
Levels of nuclear organisation
Linear DNA and Chromosome Basics
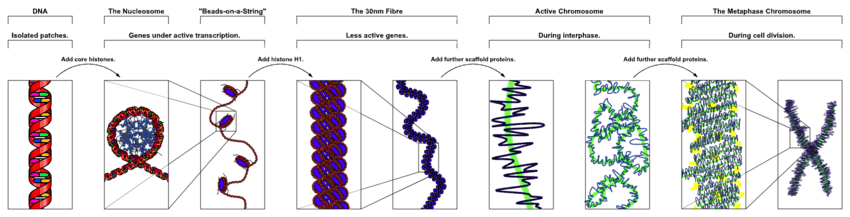
The first level of genome organization is the linear arrangement of DNA and the 3D formation of chromosomes. DNA is composed of two antiparallel strands of nucleic acids, with two bound and opposing nucleic acids referred to as DNA base pairs. In order for DNA to pack inside the tiny cell nucleus, each strand is wrapped around histones, forming nucleosome structures. These nucleosome pack together to form chromosomes. Depending on the eukaryote, there are multiple independent chromosomes of varying sizes within each nucleus - for example, humans have 46 while giraffes have 30.[14]
Within regions of the chromosome, the order of the DNA base pairs makes up specific elements for gene expression and DNA replication. Some of the more common elements include protein coding genes (containing exons and introns), noncoding DNA, enhancers, promoters, operators, origins of replication, telomeres, and centromeres. As of yet, there is not much evidence towards the importance of specific order of these elements along or between individual chromosomes. For example, the distance between an enhancer and a promoter, interacting elements that form a basis of gene expression, can range from a few hundred base pairs to 100s of kb away.[15] As well, individual enhancers can interact with a number of different promoters and the same is true for a single promoter interacting with multiple different enhancers.
However, on a larger scale, chromosomes are heterogeneous in the context of euchromatin and heterochromatin composition. As well, there is evidence of gene rich and poor regions and various domains associated with cell differentiation, active or repressed gene expression, DNA replication, and DNA recombination and repair.[16] All of these help determine chromosome territories (CTs).
DNA Looping and Chromosomal Domains
An intrinsic characteristic of chromatin fibers, DNA looping acts as the first organizational level of chromosomal folding and in turn helps regulate gene expression during interphase.
In a looping event, chromatin forms physical loops, bringing DNA regions into contact. The process is facilitated by a number of factors including architectural proteins (primarily CTCF and Cohesin), transcription factors, co-activators, and nc-RNAs. Looping can repress or activate genes, depending on the elements involved. Approximately 50% of human genes are believed to be involved in long range chromatin interactions through the process of DNA looping.[17]
Looping was first observed by Walther Flemming in 1878 when he was studying amphibian oocytes. It was not until the late 20th century when DNA looping was correlated with gene expression.[2] For example, in 1990 Mandal et al. credited DNA looping to the repression of the galactose and lactose operon when in the presence of galactose or lactose. The proteins form protein-protein and protein-DNA interactions to loop the DNA. This in turn connects the gene promoters with upstream and downstream operators, effectively repressing gene expression by blocking PIC complex assembly at the promoter and therefore preventing transcription initiation.[18]
DNA looping in gene activation typically involves the coming together of distal gene promoters and enhancers. The enhancer is able to recruit a large complex of proteins, such as the mediator, PIC complex, and other cell specific transcription factors, involved in initiating the transcription of a gene.[19]
Self-Interacting Domains
Self-interacting (or self-associating) domains are found in many organisms - in bacteria, they are referred to as Chromosomal Interacting Domains (CIDs), whereas in mammalian cells, they are called Topologically Associating Domains (TADs). Self-interacting domains can range from the 1-2 mb scale in larger organisms [20] to 10s of kb in single celled organisms.[21] What characterizes a self-interacting domain is a set of common features. The first is that self-interacting domains have a higher of ratio of chromosomal contacts within the domain than outside it. They are formed through the help of architectural proteins and contain within them many chromatin loops. This characteristic was discovered using Hi-C techniques.[17] Second, self-interacting domains correlate with regulation of gene expression. There specific domains that are associated with active transcription and other domains that repress transcription. What distinguishes whether a domain takes a particular form is dependent on which associated genes need to be active/inactive during particular phase of growth, cell cycle stage, or within a specific cell type. Cellular differentiation is determined by particular sets of genes being on or off, corresponding with the unique makeup of an individual cell’s self-interacting domains.[22] Lastly, the outside boundaries of these domains contain a higher frequency of architectural protein binding sites, regions and epigenetic marks correlated to active transcription, housekeeping genes, and short interspaced nuclear elements (SINEs).[17]
An interesting example of a subset of self-interacting domains is active chromatin hubs (ACHs). These hubs were discovered during observation of activated alpha- and beta-globin loci.[23] ACHs are formed through extensive DNA looping to form a "hub" of regulatory elements in order to coordinate the expression of a subset of genes.[24]
Lamina-Associating Domains and Nucleolar-Associating Domains
Similar to self-interacting domains, lamina-associating domains (LADs) and Nucleolar-Associating Domains (NADs) are regions of the chromosome that interact with the nuclear lamina and nucleolus, respectively.
Making up approximately 40% of the genome, LADs consist mostly of gene poor regions and span between 40kb to 30Mb in size.[25] There two known types of LADs, constitutive LADs (cLADs) and facultative LADs (fLADs). cLADs are A-T rich heterochromatin regions that remain on lamina and are seen across many types of cells and species. There is evidence that these regions are important to the structural formation of interphase chromosome. On the other hand, fLADs have varying lamina interactions and contain genes that are either activated or repressed between individual cells indicating cell-type specificity.[26] The boundaries of LADs, like self-interacting domains, are enriched in transcriptional elements and architectural protein binding sites.[25]
NADs, which constitutes 4% of the genome, share near all of the same physical characteristics as LADs. In fact, DNA analysis of these two types of domains have shown that many sequences overlap, indicating that certain regions may switch between lamina-binding and nucleolus-binding.[27] Interestingly, NADs are associated with nucleolus function. The nucleolus is the largest sub-organelle within the nucleus and is the principal site for rRNA transcription. It also acts in signal recognition particle biosynthesis, protein sequestration, and viral replication.[28] The nucleolus forms around rDNA genes from different chromosomes. However, only a subset of rDNA genes is transcribed at a time and do so by looping into the interior of the nucleolus. The rest of the genes lay on the periphery of the sub-nuclear organelle in silenced heterochromatin state.[27]
A/B Compartments
The last level of organization before full chromosome territories is the formation of A/B Compartments. A/B compartments are on the multi-Mb scale and correlate with either open and expression active, "A" compartments, or closed and expression inactive, "B" compartments, chromosomal regions.[29] A compartments tend to be gene-rich, have high GC-content, contain histone markers for active transcription, and usually displace the interior of the nucleus. As well, they are typically made up of self-interacting domains and contain early replication origins. B compartments, on the other hand, tend to be gene-poor, compact, contain histone markers for gene silencing, and lie on the nuclear periphery. They are consisted mostly of LADs and contain late replication origins.[29]
Throughout the nucleus, it has been found that A/B compartments within a chromosomal territory tend to group with respective compartments on other chromosomes, A’s with A’s and B’s with B’s. This correlates with the idea that the nucleus localizes proteins, and other factors such as long non-coding RNA (lncRNA), in regions suited for their individual roles. An example of this is the presence of multiple transcription factories throughout the nuclear interior.[30] These factories are associated with elevated levels of transcription due to the high concentration of transcription factors such as transcription protein machinery, active genes and regulatory elements, and nascent RNA. In fact, it has been revealed that roughly 95% of active genes are transcribed within transcription factories. As well, multiple genes with similar product functions or not, from the same or different chromosomes can be transcribed at same time within one factory. The last interesting characteristic of these particular foci it co-localization of genes within transcription factories are cell type dependent.[31]
Similar to domain variation during cell differentiation, A/B compartments vary between cell types. This once again supports the hypothesis that genome architecture, specific gene expression, and cell differentiation are interconnected.
Chromosome Territories
The last level of organization is the distinct positioning of individual chromosomes within the nucleus called chromosome territories (CTs). There are a few shared properties of CTs among eukaryotes. First, although chromosomal locations are not the same across cells within a population, there is some preference among individual chromosomes for particular regions. For example, large, gene-poor chromosomes are commonly located on the periphery near the nuclear lamina while smaller, gene-rich chromosomes group closer to the center of the nucleus.[32] Second, individual chromosome preference is variable among different cell types. An example from a study of spatial organization of chromosomes across multiple cell tissue conducted by Parada et al. is that the X-chromosome was found to prefer to localize in the periphery more often in liver cells than in kidney cells (Parada et al., 2004). Another conserved property of chromosome territories is that homologous chromosomes tend to be far apart from one another during cell interphase. The final characteristic is that the position of individual chromosomes during each cell cycle stays relatively the same until the start of mitosis.[33] The mechanisms and reasons behind chromosome territory characteristics is still unknown and further experimentation is needed.
References
- ↑ Cremer, T.; Cremer, M.; Hubner, B.; Strickfaden, H.; Smeets, D.; Popken, J., et al. (October 2015). "The 4D nucleome: Evidence for a dynamic nuclear landscape based on co-aligned active and inactive nuclear compartments". FEBS Letters. 589(20A): 2931–2943. doi:10.1016/j.febslet.2015.05.037.
- 1 2 Fraser, J.; Williamson, I.; Bickmore, W. A.; Dostie, J. (2015). "An overview of genome organization and how we got there: From FISH to Hi-C". Microbiol. Mol. Biol. Rev. 79(3): 347–372. doi:10.1128/MMBR.00006-15. PMC 4517094.
- ↑ Risca, V.I., and Greenleaf, W.J. (2015). "Unraveling the 3D genome: genomics tools for multiscale exploration". Trends Genet. 31(7): 357–372. doi:10.1016/j.tig.2015.03.010.
- ↑ de Wit E. and de Laat W. (2012) "A decade of 3C technologies: insights into nuclear organization". Genes Dev. 26(1): 11–24. doi:10.1101/gad.179804.111.
- ↑ Gaj, T.; Gersbach, C.A.; Barbas, C.F. (2013). "ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering". Trends Biotechnol 31(7): 397–405. doi:10.1016/j.tibtech.2013.04.004.
- ↑ Gómez-Díaz, E; Corces, VG (November 2014). "Architectural proteins: regulators of 3D genome organization in cell fate". Trends in Cell Biology. 24 (11): 703–11. doi:10.1016/j.tcb.2014.08.003. PMID 25218583.
- ↑ Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ (1997). "Crystal structure of the nucleosome core particle at 2.8 A resolution". Nature389 (6648): 251–60.
- ↑ Phillips, T. & Shaw, K. (2008) Chromatin Remodeling in Eukaryotes. Nature Education 1(1):209
- ↑ Wiechens N, Singh V, Gkikopoulos T, Schofield P, Rocha S, Owen-Hughes T. (2016) The Chromatin Remodelling Enzymes SNF2H and SNF2L Position Nucleosomes adjacent to CTCF and Other Transcription Factors. PLoS Genet. 2016 Mar 28
- ↑ Chaumeil J and Skok JA (April 2012). "The role of CTCF in regulating V(D)J recombination". Curr. Opin. Immunol. 24 (2): 153–9.
- ↑ Peters JM, Tedeschi A, Schmitz J (2008) "The cohesin complex and its roles in chromosome biology". Genes Dev 22(22):3089–3114.
- ↑ Mehta G, Kumar R, Srivastava S, Ghosh SK (2013). "Cohesin: Functions beyond sister chromatid cohesion". FEBS Letters.
- ↑ Nasmyth, K. & Haering, C. H. (2009) "Cohesin: its roles and mechanisms." Annu. Rev. Genet. 43, 525–558.
- ↑ Huang L, Nesterenko A, Nie W, Wang J, Su W, Graphodatsky AS, Yang F: Karyotypic evolution of giraffes (Giraffa camelopardalis) revealed by cross-species chromosome painting with Chinese muntjac (Muntiacus reevesi) and human (Homo sapiens) paints. Cytogenet Genome Res. 2008, 122: 132-138.
- ↑ Matthews, KS (March 1992). "DNA looping". Microbiol Rev. 56(1):123–136.
- ↑ Federico C, Scavo C, Cantarella CD, Motta S, Saccone S, et al. (2006). "Gene-rich and gene-poor chromosomal regions have different locations in the interphase nuclei of cold-blooded vertebrates". Chromosoma 115: 123–128. doi:10.1007/s00412-005-0039-z.
- 1 2 3 Jin F., Li Y., Dixon J.R., Selvaraj S., Ye Z., Lee A.Y., et al. (2013) "A high-resolution map of the three-dimensional chromatin interactome in human cells" Nature, 503 (2013), pp. 290–294
- ↑ Mandal N., Su W., Haber R., Adhya S., Echols H. (1990) "DNA looping in cellular repression of transcription of the galactose operon." Genes Dev. 4:410–418.
- ↑ Liu Z, Merkurjev D, Yang F, Li W, Oh S, Friedman MJ, et al. (2014) "Enhancer activation requires trans-recruitment of a mega transcription factor complex." Cell. 2014;159(2):358–373
- ↑ Dixon J.R., Selvaraj S., Yue F., Kim A., Li Y., Shen Y., et al. (2012) "Topological domains in mammalian genomes identified by analysis of chromatin interactions" Nature, 485 (2012), pp. 376–380
- ↑ Le T.B., Imakaev M.V., Mirny L.A., Laub M.T. (2013) "High-resolution mapping of the spatial organization of a bacterial chromosome" Science, 342 (2013), pp. 731–734
- ↑ Li G., Ruan X., Auerbach R.K., Sandhu K.S., Zheng M., Wang P., et al. (2012) "Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation" Cell, 148 (2012), pp. 84–98
- ↑ Tolhuis B., Palstra R.J., Splinter E., Grosveld F., de Laat W. (2002) "Looping and interaction between hypersensitive sites in the active beta-globin locus" Mol Cell, 10 (2002), pp. 1453–1465
- ↑ de Laat W and Grosveld F. (2003) "Spatial organization of gene expression: the active chromatin hub". Chromosome Res. 2003;11:447–459.
- 1 2 Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W, van Steensel B (June 2008). "Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions". Nature 453 (7197): 948–51.
- ↑ Meuleman W. (2013) "Constitutive nuclear lamina– genome interactions are highly conserved and associated with A/T-rich sequence". Genome Res. 23, 270–280.
- 1 2 van Koningsbruggen S., Gierlinski M., Schofield P., Martin D., Barton G.J., Ariyurek Y. (2010) "High-resolution whole-genome sequencing reveals that specific chromatin domains from most human chromosomes associate with nucleoli" Mol Biol Cell, 21 (2010), pp. 3735–3748
- ↑ Matheson T.D., Kaufman P.D. (June 2016). "Grabbing the genome by the NADs". Chromosoma. 125(3): 361–371. doi:10.1007/s00412-015-0527-8
- 1 2 Lieberman-Aiden E., van Berkum N.L., Williams L., Imakaev M., Ragoczy T., Telling A. (2009) "Comprehensive mapping of long-range interactions reveals folding principles of the human genome" Science, 326 (2009), pp. 289–293
- ↑ Cook P.R. (2010) "A model for all genomes: the role of transcription factories". J Mol Biol, 395 (2010), pp. 1–10
- ↑ Buckley M.S., Lis J.T. (2014) "Imaging RNA Polymerase II transcription sites in living cells". Curr Opin Genet Dev, 25 (2014), pp. 126–130
- ↑ Croft J.A., Bridger J.M., Boyle S., Perry P., Teague P., Bickmore W.A. (1999) "Differences in the localization and morphology of chromosomes in the human nucleus". J Cell Biol, 145 (1999), pp. 1119–1131
- ↑ Walter J., Schermelleh L., Cremer M.,. Tashiro S, Cremer T. (2003) "Chromosome order in HeLa cells changes during mitosis and early G1, but is stably maintained during subsequent interphase stages". J Cell Biol, 160 (2003), pp. 685–697