Etynodiol diacetate
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
G03DC06 (WHO) G03AA01 (WHO) G03FA06 (WHO) (with an estrogen) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms | 3β-Hydroxynorethisterone 3,17-diacetate[1] |
| CAS Number |
297-76-7 8056-92-6 |
| PubChem (CID) | 92709270 |
| DrugBank | DB00823 |
| ChemSpider | 8913 |
| Chemical and physical data | |
| Formula | C24H32O4 |
| Molar mass | 384.509 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Etynodiol diacetate (sold as Continuin, Femulen, Luteonorm, Luto-Metrodiol, and Metrodiol), or ethynodiol diacetate (USAN, BAN, JAN), also known as norethindrol diacetate or 3β-hydroxynorethisterone diacetate,[2] is a steroidal progestin of the 19-nortestosterone group which is used as a hormonal contraceptive.[3][4] It is the 3β,17β-diacetate ester of etynodiol,[3][4] and acts as a rapidly converted prodrug to norethisterone,[5] with etynodiol occurring as an intermediate.[6][7] Etynodiol diacetate has weak androgenic activity,[8][9] and, unlike most progestins but similarly to norethisterone and noretynodrel,[10] also has some estrogenic activity.[9][11]
Pharmacology
Etynodiol diacetate is virtually inactive in terms of affinity for the progesterone and androgen receptors[6] and acts as a prodrug of etynodiol,[7] which in turn is a prodrug of norethisterone.[5] Upon oral administration and during first-pass metabolism in the liver, etynodiol diacetate is rapidly converted by esterases into etynodiol,[7] which is followed by oxygenation of the C3 hydroxyl group to produce norethisterone.[5]
Chemistry
Synthesis
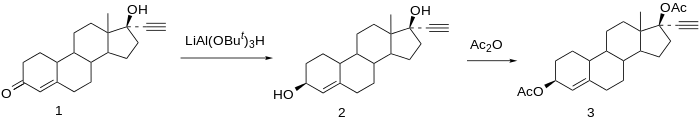
Reduction of norethisterone (1) affords the 3,17-diol. The 3β-hydroxy compound is the desired product; since reactions at C-3 do not show nearly the stereoselectivity as those at C-17 by virtue of the relative lack of stereo-directing proximate substituents, the formation of the desired isomer is engendered by use of a bulky reducing agent, Lithium tri-tert-butoxyaluminum hydride. Acetylation of the 3β,17β-diol affords Etynodiol diacetate (3), one of the most potent oral progestins.[12]
See also
References
- ↑ Schindler, Adolf E; Campagnoli, Carlo; Druckmann, René; Huber, Johannes; Pasqualini, Jorge R; Schweppe, Karl W; Thijssen, Jos H.H (2003). "Classification and pharmacology of progestins". Maturitas. 46: 7–16. doi:10.1016/j.maturitas.2003.09.014. ISSN 0378-5122.
- ↑ Donna Shoupe; Florence P. Haseltine (6 December 2012). Contraception. Springer Science & Business Media. pp. 21–. ISBN 978-1-4612-2730-4.
- 1 2 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 522–. ISBN 978-1-4757-2085-3.
- 1 2 Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 422. ISBN 978-3-88763-075-1. Retrieved 30 May 2012.
- 1 2 3 IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 146–. ISBN 978-92-832-1291-1.
- 1 2 Hammerstein J (1990). "Prodrugs: advantage or disadvantage?". Am. J. Obstet. Gynecol. 163 (6 Pt 2): 2198–203. PMID 2256526.
- 1 2 3 Stanczyk FZ (2002). "Pharmacokinetics and potency of progestins used for hormone replacement therapy and contraception". Rev Endocr Metab Disord. 3 (3): 211–24. PMID 12215716.
- ↑ Armen H. Tashjian; Ehrin J. Armstrong (21 July 2011). Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Lippincott Williams & Wilkins. pp. 523–. ISBN 978-1-4511-1805-6.
- 1 2 Kenneth L. Becker (24 April 2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. p. 1004. ISBN 978-0-7817-1750-2. Retrieved 30 May 2012.
- ↑ Benno Clemens Runnebaum; Thomas Rabe; Ludwig Kiesel (6 December 2012). Female Contraception: Update and Trends. Springer Science & Business Media. pp. 36–. ISBN 978-3-642-73790-9.
- ↑ Allan H. Goroll; Albert G. Mulley (27 January 2009). Primary Care Medicine: Office Evaluation and Management of the Adult Patient. Lippincott Williams & Wilkins. p. 876. ISBN 978-0-7817-7513-7. Retrieved 30 May 2012.
- 1 2 Klimstra, P.; Colton, F. (1967). "The synthesis of 3β-hydroxyestr-4-en-17-one and 3β-hydroxiandrost-4-en-17-one". Steroids. 10 (4): 411. doi:10.1016/0039-128X(67)90119-5.
- ↑ Sondheimer, F.; Klibansky, Y. (1959). "Synthesis of 3β-hydroxy analogues of steroidal hormones, a biologically active class of compounds". Tetrahedron. 5: 15. doi:10.1016/0040-4020(59)80066-1.