Etynodiol
 | |
| Clinical data | |
|---|---|
| ATC code | G03DC06 (WHO) |
| Identifiers | |
| |
| CAS Number | 1231-93-2 |
| PubChem (CID) | 14687 |
| ChemSpider | 14017 |
| Chemical and physical data | |
| Formula | C20H28O2 |
| Molar mass | 300.435 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Etynodiol (INN), or ethynodiol (BAN), also known as 3β-hydroxynorethisterone,[1] is a steroidal progestin related to norethisterone which was never marketed.[2][3] A diacylated derivative, etynodiol diacetate, is used as a hormonal contraceptive.[2][3] While etynodiol is sometimes used as a synonym for etynodiol diacetate, what is usually being referred to is actually etynodiol diacetate and not etynodiol.
Pharmacology
Etynodiol is a prodrug of norethisterone, and is converted immediately and completely into norethisterone.[4][5][6] In addition, etynodiol is an intermediate in the conversion of the prodrug lynestrenol into norethisterone.[7]
Chemistry
Etynodiol is a 19-nortestosterone derivative. Structurally, it is almost identical to norethisterone and lynestrenol, differing only in its C3 substituent. Whereas norethisterone has a ketone at C3 and lynestrenol has no substituent at C3, etynodiol has a hydroxyl group at the position.
Synthesis
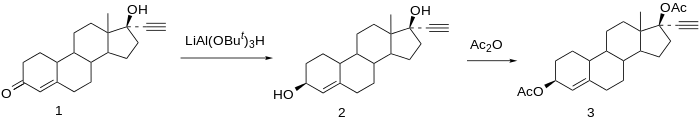
See also
References
- ↑ Schindler, Adolf E; Campagnoli, Carlo; Druckmann, René; Huber, Johannes; Pasqualini, Jorge R; Schweppe, Karl W; Thijssen, Jos H.H (2003). "Classification and pharmacology of progestins". Maturitas. 46: 7–16. doi:10.1016/j.maturitas.2003.09.014. ISSN 0378-5122.
- 1 2 F.. Macdonald (1997). Dictionary of Pharmacological Agents. CRC Press. p. 1454. ISBN 978-0-412-46630-4. Retrieved 12 May 2012.
- 1 2 Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 422. ISBN 978-3-88763-075-1. Retrieved 30 May 2012.
- ↑ Donna Shoupe; Florence P. Haseltine (6 December 2012). Contraception. Springer Science & Business Media. pp. 21–. ISBN 978-1-4612-2730-4.
- ↑ Bhattacharya (1 January 2003). Pharmacology, 2/e. Elsevier India. pp. 378–. ISBN 978-81-8147-009-6.
- ↑ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 146–. ISBN 978-92-832-1291-1.
- ↑ Hammerstein J (1990). "Prodrugs: advantage or disadvantage?". Am. J. Obstet. Gynecol. 163 (6 Pt 2): 2198–203. PMID 2256526.
- ↑ Klimstra, P.; Colton, F. (1967). "The synthesis of 3β-hydroxyestr-4-en-17-one and 3β-hydroxiandrost-4-en-17-one". Steroids. 10 (4): 411. doi:10.1016/0039-128X(67)90119-5.
- ↑ Sondheimer, F.; Klibansky, Y. (1959). "Synthesis of 3β-hydroxy analogues of steroidal hormones, a biologically active class of compounds". Tetrahedron. 5: 15. doi:10.1016/0040-4020(59)80066-1.