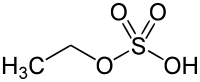
Ethyl sulfate
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Ethyl hydrogen sulfate | |
| Other names
Ethyl sulfate; Sulfovinic acid; Ethyl bisulfate; Ethoxysulfonic acid; Ethyl sulphate | |
| Identifiers | |
| 540-82-9 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 5782 |
| ECHA InfoCard | 100.007.963 |
| PubChem | 6004 |
| |
| |
| Properties | |
| C2H6O4S | |
| Molar mass | 126.13 g·mol−1 |
| Density | 1.46 g/cm³ |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Ethyl sulfate (IUPAC name: ethyl hydrogen sulfate), also known as sulfovinic acid, is an organic chemical compound used as an intermediate in the production of ethanol from ethylene. It is the ethyl ester of sulfuric acid.
History
This substance was studied alongside ether for the first time by German alchemist August Siegmund Frobenius in 1730,[1] subsequently by French chemists Fourcroy in 1797 and Gay-Lussac in 1815.[2][3] Swiss scientist Nicolas-Théodore de Saussure also studied it in 1807.[4] In 1827, French chemist and pharmacist Félix-Polydore Boullay (1806-1835) along with Jean-Baptiste André Dumas noted the role of ethyl sulfate in the preparation of diethyl ether from sulfuric acid and ethanol.[5][6] Further studies by the German chemist Eilhard Mitscherlich and the Swedish chemist Jöns Berzelius suggested sulfuric acid was acting as a catalyst, this eventually led to the discovery of sulfovinic acid as an intermediate in the process.[7][8] The advent of electrochemistry by Italian physicist Alessandro Volta and English chemist Humphry Davy in the 1800s confirmed ether and water were formed by the reaction of sub-stoichiometric amounts of sulfuric acid on ethanol and that sulfovinic acid was formed as an intermediate in the reaction.[9]
Production
Ethyl sulfate can be produced in a laboratory setting by reacting ethanol with sulfuric acid under a gentle boil, while keeping the reaction below 140 °C. The sulfuric acid must be added dropwise or the reaction must be actively cooled because the reaction itself is highly exothermic.
- CH3CH2OH + H2SO4 → CH3-CH2-O-SO3H + H2O
If the temperature exceeds 140 °C, the ethyl sulfate product tends to react with residual ethanol starting material, producing diethyl ether. If the temperature exceeds 170 °C in a considerable excess of sulfuric acid, the ethyl sulfate breaks down into ethylene and sulfuric acid.[10][11]
Reaction
The mechanism of the formation of ethyl sulfate, diethyl ether, and ethylene is based on the reaction between ethanol and sulfuric acid, which involves protonation of the ethanolic oxygen to form the oxonium ion.[11]
Salts
Ethyl sulfate can exist in salt forms, such as sodium ethyl sulfate, potassium ethyl sulfate, and calcium ethyl sulfate. The salt can be formed by adding the according carbonate, or bicarbonate salt. As an example, ethyl sulfate and potassium carbonate forms potassium ethyl sulfate and potassium bicarbonate.[11]
- CH3-CH2-O-SO3H + K2CO3 → CH3-CH2-O-SO3K + KHCO3
See also
References
- ↑ Dr. Frobenius (1730) "An account of a spiritus vini æthereus, together with several experiments tried," Philosophical Transactions of the Royal Society (of London), 36 (413) : 283–289.
- ↑ Fourcroy, A.F. and Vauquelin, L.N. (1797) "Sur l'action de l'acide sulfurique sur l'alcool et de la formation de l'éther" (On the action of sulfuric acid on alcohol and on the formation of ether), Annales de Chimie, 23 : 203-215.
- ↑ Gay-Lussac, L.J. (1815) "Sur l'analyse de l'alcool et de l'éther sulfurique et sur les produits de la fermentation" (On the analysis of alcohol and sulfuric ether and on the products of fermentation), Annales de Chimie, 95 : 311-318.
- ↑ Théodore de Saussure (1807) "Mémoire sur la composition de l'alcohol et de l'éther sulfurique," Journal de physique, de chimie, d'histoire naturelle et des arts, 64 : 316–354.
- ↑ Dumas, J-B and Boullay, P. (1827) "Mémoire sur la formation de l'éther sulfurique," Annales de Chimie et de Physique, 36 : 294-316.
- ↑ Wisniak, Jaime (2010). "Félix-Polydore Boullay" (PDF). Revista CENIC Ciencias Químicas. 41 (1): 59–66.
- ↑ E. Mitscherlich (1834) "Ueber die Aetherbildung" (On the formation of ether), Annalen der Physik und Chemie, 31 (18) : 273-282.
- ↑ J. J. Berzelius, Årsberättelsen om framsteg i fysik och kemi [Annual report on progress in physics and chemistry], (Stockholm, Sweden: Royal Swedish Academy of Sciences, 1835). After reviewing Eilhard Mitscherlich's research on the formation of ether, Berzelius coins the word katalys (catalysis) on page 245:
Original: Jag skall derföre, för att begagna en i kemien välkänd härledning, kalla den kroppars katalytiska kraft, sönderdelning genom denna kraft katalys, likasom vi med ordet analys beteckna åtskiljandet af kroppars beståndsdelar medelst den vanliga kemiska frändskapen.
Translation: I shall, therefore, to employ a well-known derivation in chemistry, call [the catalytic] bodies [i.e., substances] the catalytic force and the decomposition of [other] bodies by this force catalysis, just as we signify by the word analysis the separation of the constituents of bodies by the usual chemical affinities.
- ↑ "History of Ether". The Composition and Structure of Ether. Retrieved September 7, 2005.
- ↑ Julius B. Cohen (1930). Practical Organic Chemistry (preparation 5). Macmillan.
- 1 2 3 Frederick George Mann and Bernard Charles Saunders (1960). Practical Organic Chemistry (Preparations, The Interaction of Ethanol and Sulfuric acid). Longman Inc.