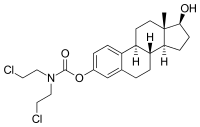
Estramustine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Emcyt |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608046 |
| License data |
|
| Pregnancy category | |
| ATC code | L01XX11 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 75%[1] |
| Metabolism | Hepatic to estradiol and estrone[1] |
| Biological half-life | 15-24 hours[1] |
| Excretion | Faeces (2.9-4.8%)[1] |
| Identifiers | |
| |
| CAS Number |
2998-57-4 |
| PubChem (CID) | 259331 |
| DrugBank |
DB01196 |
| ChemSpider |
227635 |
| UNII |
35LT29625A |
| KEGG |
D04066 |
| ChEBI |
CHEBI:4868 |
| ChEMBL |
CHEMBL1575 |
| Chemical and physical data | |
| Formula | C23H31Cl2NO3 |
| Molar mass | 440.403 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Estramustine (INN, USAN, BAN) (brand names Emcyt, Estracit), in salt form as estramustine phosphate sodium (USAN) (or estramustine sodium phosphate (BAN)) or estramustine phosphate meglumine, is an alkylating antineoplastic agent (that is, a chemotherapeutic) that is used in the treatment of prostate cancer throughout the world.[2] It is a derivative of estradiol, an estrogen, with a nitrogen mustard-carbamate ester moiety attached. The drug has been withdrawn from a number of markets, including Australia, Brazil, Ireland, and Norway.[3]
Clinical uses
Estramustine is indicated, in the US, for the palliative treatment of metastatic and/or progressive prostate cancer.[1] Whereas in the UK it is indicated for the treatment of unresponsive or relapsing prostate cancer.[4][5][6][7]
Adverse effects
Adverse effects by frequency:[1][4]
Very common (>10% frequency):
- Oedema
- Dyspnoea
- Nausea
- Diarrhoea
- Breast tenderness and enlargement
Common (1-10% frequency):
- Lethargy
- Insomnia
- Congestive heart failure
- Easy bruising
- Anorexia
- Vomiting
- Itchiness
- Dry skin
- Impotence
- Leg cramps
- Decreased testosterone
- Impaired liver function
- Blood clots and complications thereof (including stroke, heart attacks, pulmonary embolism and thrombophlebitis)
Rare (<0.1% frequency):
- Anaemia
- Leucopenia
- Thrombocytopenia
- Confusion
- Depression
- Headache
- Lethargy
- Muscle weakness
- Angiooedema, occurs most commonly when used in combination with ACE inhibitors.
Unlike other nitrogen mustards it seldom produces significant GI or haematologic toxicity such as myelosuppression,[5] the major drug toxicity-related cause of drug discontinuation is thromboembolism (blood clots).[8]
Contraindications
It is contraindicated when used in children, patients hypersensitive to estradiol or nitrogen mustards, those with peptic ulcer (an ulcer in the digestive tract), those with severely compromised liver function, those with weak heart muscle (also known as myocardial insufficiency) and those with thromboembolic disorders or complications related to fluid retention.[4]
Interactions
Estrogen mimics like estramustine have been reported to increase the toxicity and therapeutic efficacy of tricyclic antidepressants like amitriptyline and imipramine.[4] Dairy products and other products containing calcium, aluminium and magnesium have been reported to reduce the absorption of estramustine from the gastrointestinal tract hence reducing bioavailability.[4] There may be an increased risk of angiooedema in those concurrently taking ACE inhibitors.[4]
Mechanism of action
Estramustine acts by two mechanisms: 1) antigonadotropic effects which suppress gonadal androgen production (caused by its estrogenic activity); and 2) direct cytotoxic activity via inhibition of microtubules (caused by its nitrogen mustard moiety).[9] In regards to the latter, there is depolymerization of the microtubules (which estramustine achieves by binding to microtubule associated proteins); this arrests prostate cancer cells in the G2/M phase of the cell cycle.[5][6][7] The drug is selectively taken up by prostate cells and hence produces minimal cytotoxic effects on healthy tissue.[5]
Pharmacokinetics
Estramustine is delivered as an oral capsule. It is readily taken up from the gastrointestinal tract and then rapidly dephosphorylated from estramustine phosphate to estramustine.[5] Estramustine is then partially oxidized to estromustine.[5] Some estramustine and estromustine undergoes hydrolysis at the ester bond in the liver to form estradiol, estrone and normustine.[5] Milk, calcium, aluminium and magnesium supplements may reduce bioavailability.[4]
| Estramustine | Estromustine | Estradiol | Estrone | Normustine |
|---|---|---|---|---|
 |  | 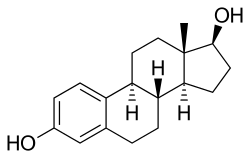 | 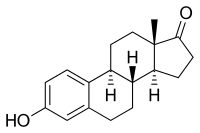 |  |
See also
References
- 1 2 3 4 5 6 "Emcyt (estramustine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 8 February 2014.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 406–407. ISBN 978-3-88763-075-1.
- ↑ Sweetman, S, ed. (12 February 2013). "Estramustine Sodium Phosphate". Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. Retrieved 8 February 2014.
- 1 2 3 4 5 6 7 "Estracyt Capsules - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Pfizer Limited. 12 August 2013. Retrieved 8 February 2014.
- 1 2 3 4 5 6 7 Perry, CM; McTavish, D (July 1995). "Estramustine Phosphate Sodium". Drugs & Aging. 7 (1): 49–74. doi:10.2165/00002512-199507010-00006. PMID 7579781.
- 1 2 Bergenheim, AT; Henriksson, R (February 1998). "Pharmacokinetics and pharmacodynamics of estramustine phosphate.". Clinical pharmacokinetics. 34 (2): 163–72. doi:10.2165/00003088-199834020-00004. PMID 9515186.
- 1 2 Simpson, D; Wagstaff, AJ (2003). "Estramustine Phosphate Sodium". American Journal of Cancer. 2 (5): 373–390. doi:10.2165/00024669-200302050-00013.
- ↑ Fizazi K, Le Maitre A, Hudes G, Berry WR, Kelly WK, Eymard JC, Logothetis CJ, Pignon JP, Michiels S (2007). "Addition of estramustine to chemotherapy and survival of patients with castration-refractory prostate cancer: a meta-analysis of individual patient data". Lancet Oncol. 8 (11): 994–1000. doi:10.1016/S1470-2045(07)70284-X. PMID 17942366.
- ↑ Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 540–. ISBN 978-3-642-60107-1.