Erythrose
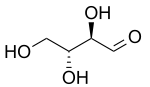 D-Erythrose | |
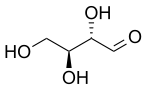 L-Erythrose | |
| Names | |
|---|---|
| IUPAC names
(2R,3R)-2,3,4-Trihydroxybutanal (D) (2S,3S)-2,3,4-Trihydroxybutanal (L) | |
| Identifiers | |
| 583-50-6 (D) 533-49-3 (L) | |
| 3D model (Jmol) | (D): Interactive image (L): Interactive image |
| ChEBI | CHEBI:27904 |
| ChemSpider | 84990 (D) |
| ECHA InfoCard | 100.008.643 |
| PubChem | 94176 (D) |
| |
| |
| Properties | |
| C4H8O4 | |
| Molar mass | 120.10 g·mol−1 |
| Appearance | Light yellow syrup |
| Very soluble | |
| Hazards | |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Erythrose is a tetrose carbohydrate with the chemical formula C4H8O4. It has one aldehyde group, and so is part of the aldose family. The natural isomer is D-erythrose.
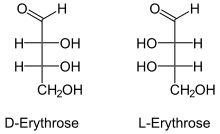
Fischer projections
Erythrose was first isolated in 1849 from rhubarb by the French pharmacist Louis Feux Joseph Garot (1798-1869),[2] and was named as such because of its red hue in the presence of alkali metals (ἐρυθρός, "red").[3][4]
Erythrose 4-phosphate is an intermediate in the pentose phosphate pathway[5] and the Calvin cycle.[6]
Oxidative bacteria can be made to use erythrose as its sole energy source.[7]
See also
References
- ↑ Merck Index, 11th Edition, 3637
- ↑ Obituary of Garot (1869) Journal de pharmacie et de chimie, 4th series, 9 : 472-473.
- ↑ Garot (1850) "De la matière colorante rouge des rhubarbes exotiques et indigènes et de son application (comme matière colorante) aux arts et à la pharmacie" (On the red coloring material of exotic and indigenous rhubarb and on its application (as a coloring material) in the arts and in pharmacy), Journal de Pharmacie et de Chimie, 3rd series, 17 : 5-19. Erythrose is named on p. 10: "Celui que je propose, sans y attacher toutefois la moindre importance, est celui d'érythrose, du verbe grec 'ερυθραινω, rougir (1)." (The one [i.e., name] that I propose, without attaching any importance to it, is that of erythrose, from the Greek verb ερυθραινω, to redden (1).)
- ↑ Wells, David Ames; Cross, Charles Robert; Bliss, George; Trowbridge, John; Nichols, William Ripley; Kneeland, Samuel (1851). Annual of Scientific Discovery. Boston: Gould, Kendall, and Lincoln. p. 211. Retrieved 11 December 2014.
- ↑ Kruger, Nicholas J; von Schaewen, Antje (June 2003). "The oxidative pentose phosphate pathway: structure and organisation". Current Opinion in Plant Biology. 6 (3): 236–246. doi:10.1016/S1369-5266(03)00039-6. Retrieved 11 December 2014.
- ↑ Schwender, Jörg; Goffman, Fernando; Ohlrogge, John B.; Shachar-Hill, Yair (9 December 2004). "Rubisco without the Calvin cycle improves the carbon efficiency of developing green seeds". Nature. 432 (7018): 779–782. doi:10.1038/nature03145. Retrieved 11 December 2014.
- ↑ Hiatt, Howard H; Horecker, B L (13 October 1955). "D-erythrose metabolism in a strain of Alcaligenes faecalis". Journal of Bacteriology. 71 (6): 649–654. Retrieved 11 December 2014.
This article is issued from Wikipedia - version of the 8/21/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
