Elevated plus maze
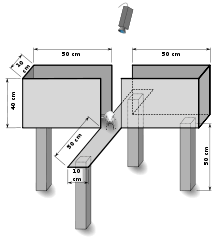
The elevated plus maze (EPM) is a test measuring anxiety in laboratory animals that usually uses rodents as a screening test for putative anxiolytic or anxiogenic compounds and as a general research tool in neurobiological anxiety research such as PTSD and TBI.[1] The model is based on the test animal's aversion to open spaces and tendency to be thigmotaxic. In the EPM, this anxiety is expressed by the animal spending more time in the enclosed arms.
Method

The test uses an elevated, plus-shaped (+) apparatus with two open and two enclosed arms. The behavioral model is based on the general aversion of rodents to open spaces. This aversion leads to the behavior termed thigmotaxis, a preference for remaining in enclosed spaces or close to the edges of a bounded space. In the EPM, this translates into the animals limiting their movement to the enclosed arms.[2][3][4][5]
Anxiety reduction is indicated in the plus-maze by an increase in the proportion of time spent in the open arms (time in open arms/total time in open or closed arms), and an increase in the proportion of entries into the open arms (entries into open arms/total entries into open or closed arms). The total number of arm entries and number of closed-arm entries are sometimes used as measures of general activity.[6] The relationship between the EPM and other tests of exploratory activity (open-field and emergence) have been analyzed in two mouse strains.[7]
Controversy
While EPM is the most commonly employed animal behavioral model of anxiety, there are several issues concerning the validity of the model. Classical clinical anxiolytics, such as benzodiazepines (e.g., Valium), do reduce measures of anxiety in EPM. However, more novel compounds, such as 5-HT1A agonists (e.g., Buspar) give mixed results. Selective serotonin reuptake inhibitors and tricyclic antidepressants, which are commonly employed in clinical settings to treat anxiety disorders, also do not lead to a stable anxiolytic effect on EPM.[5] This raises the possibility that EPM is a suitable model for testing GABA-related compounds, such as benzodiazepines or direct GABAA agonists, but not for other drugs. Despite this, the model is commonly employed for screening putative anxiolytics and for general research into the brain mechanisms of anxiety,[8] likely due to the ease of employment and the vast number of studies already in the literature.
Variations
Elevated zero maze
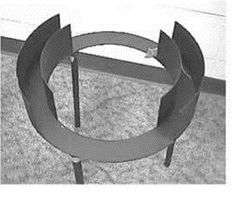
The elevated zero maze (EZM) is an elevated circular runway with alternating open light areas and enclosed dark areas.[9][10] The continuous nature of this apparatus eliminates the problem of the EPM in how to account for the animal's presence in the center area of the EPM. In the EPM test, animals may spend up to 30% of their time in the ambiguous central start area or return to it often, making it difficult to evaluate the biological significance of anxiety related behaviour. Animal will return to the central area because they are habituated to that area and associate it with being "safe".[11]
Untreated rodents show a higher exploration of open areas in the EZM then in the EPM. This could indicate the EPM inhibits exploration, but the fact that rodents spent time in the central zone of the EPM needs to be taken into account. The EZM is more sensitive to changes then the EPM due to the baseline level in the EZM being lower than the EPM.[9]
Elevated T maze
The elevated T maze (ETM) has three arms in the shape of the letter "T". One arm is closed and perpendicular to the other two arms which are open. This test is designed to observe anxiety effects and how it affects learning. The rodent is placed on the enclosed arm and allowed to explore. The trial ends when the rodents sets all four paws in on the open arms. Rats are allowed multiple trials until they learn to stay in the open arm for 300 seconds. This is a measurement of inhibitory avoidance.Depending on what the rodents were treated with during the training sessions, they would learn at different rates giving information on how the brain stores memories.[12] This test can be used to assess long term memory. When a rodent has been sufficiently trained, researchers will test the rodent again after a week to observe if the rodent still remembers to stay in the enclosed arm.
Plus-maze discriminative avoidance test
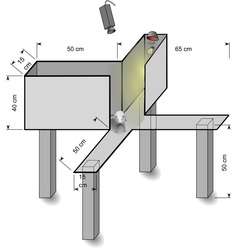
Like the standard EPM, the apparatus used in the plus-maze discriminative avoidance test (PMDAT) has four arms. This test has been used to investigate interactions between aversive memory and anxiety responses in rodents. The apparatus has two open arms opposite to two enclosed arms. In this test, one of the enclosed arms is paired with aversive stimuli (e.g. bright light, loud white noise). During training, animals are placed in the apparatus facing the intercept between the open arms. Each time the animal enters the aversive enclosed arm, the aversive stimulus is presented until the animal leaves the arm. Upon a second exposure to the maze (e.g. 24 h later) the aversive stimuli is no longer presented. Retention of the aversive memory is assessed based on the relative time spent in the non-aversive arm compared to the previously aversive arm and anxiety behavior is calculated based on the time spent in the open arms during the training session.[13]
See also
External links
- Elevated Plus Maze Academic Blog
- Video of the elevated plus maze
References
- ↑ Ojo, J; Mouzon, B (May 2016). "Chronic Repetitive Mild Traumatic Brain Injury Results in Reduced Cerebral Blood Flow, Axonal Injury, Gliosis, and Increased T-Tau and Tau Oligomers.". J Neuropathol Exp Neurol. doi:10.1093/jnen/nlw035. PMID 27251042.
- ↑ Pellow, S., Chopin, P., File, S.E. and Briley, M. (1985). "Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat". J. Neurosci. Methods. 14: 149–67. doi:10.1016/0165-0270(85)90031-7.
- ↑ Treit, D., Menard, J. and Royan, C. (1993). "Anxiogenic stimuli in the elevated plus-maze". Pharmacol. Biochem. Behav. 44: 463–469. doi:10.1016/0091-3057(93)90492-c.
- ↑ Rodgers, R.J. (1997). "Animal models of anxiety: where next?". Behav. Pharmacol. 8: 477–496. doi:10.1097/00008877-199711000-00003.
- 1 2 Carobrez, A.P.; Bertoglio, L.J. (2005). "Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on". Neurosci. Biobehav. Rev. 29: 1193–1205. doi:10.1016/j.neubiorev.2005.04.017.
- ↑ Hogg S.A. (1996). "Review of the validity and variability of the elevated plus-maze as an animal model of anxiety". Pharmacol. Biochem. Behav. 54: 21–30. doi:10.1016/0091-3057(95)02126-4.
- ↑ Lalonde, R; Strazielle C (2008). "Relations between open-field, elevated plus-maze, and emergence tests as displayed by C57/BL6J and BALB/c mice". Journal of Neuroscience Methods. 171 (1): 48–52. doi:10.1016/j.jneumeth.2008.02.003. PMID 18358538.
- ↑ Engin, E.; Treit, D. (2008). "The effects of intra-cerebral drug infusions on animals' unconditioned fear reactions: A systematic review.". Prog. Neuropsychopharmacol. Biol. Psychiatry. doi:10.1016/j.pnpbp.2008.03.020.
- 1 2 Braun, Amanda A; Skelton, Matthew R; Vorhees, Charles V; Williams, Michael Y (2011). "Comparison of the elevated plus and elevated zero mazes in treated and untreated male Sprague-Dawley rats: Effects of anxiolytic and anxiogenic agents". Pharmacol Biochem Behav. 97 (3): 406–415. doi:10.1016/j.pbb.2010.09.013.
- ↑ Campos, Alline C; Fogaca, Manoela V; Aguiar, Daniele C; Guimaraes, Francisco S (2013). "Animal models of anxiety disorders and stress". Revista Brasileira de Psiquiatria. 35: 101–111.
- ↑ Bailey, KR; Crawley, JN (2009). Methods of Behavior Analysis in Neuroscience (2nd ed.). Boca Raton (FL): CRC Press/Taylor & Francis. Retrieved 13 April 2016.
- ↑ Asth, Laila; Rachetti, Vanessa; Gavioli, Elaine C; Andre, Eunice; Lobao-Soares, Bruno (2012). "The elevated T-maze task as an animal model to simultaneously investigate the effects of drugs on long-term memory and anxiety in mice". Brain Research Bulletin. 87: 526–533. doi:10.1016/j.brainresbull.2012.02.008.
- ↑ Leão, Anderson; Medeiros, Andre; Apolinario, Genedy; Cabral, Alicia; Ribeiro, Alessandra; Barbosa, Flavio; Silva, Regina (2016). "Hippocampal-dependent memory in the plus-maze discriminative avoidance task: The role of spatial cues and CA1 activity". Behavioural Brain Research. 304: 24–33. doi:10.1016/j.bbr.2016.02.012.