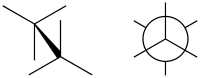
Eclipsed conformation
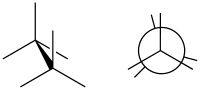
(image right in Newman projection)
In chemistry an eclipsed conformation is a conformation in which two substituents X and Y on adjacent atoms A, B are in closest proximity, implying that the torsion angle X–A–B–Y is 0°.[1] Such a conformation exists in any open chain, single chemical bond connecting two sp3-hybridised atoms, and it is normally a conformational energy maximum. This maximum is often explained by steric hindrance, but its origins sometimes actually lie in hyperconjugation (as when the eclipsing interaction is of two hydrogen atoms).
In the example of ethane in Newman projection it shows that rotation around the carbon-carbon bond is not entirely free but that an energy barrier exists. The ethane molecule in the eclipsed conformation is said to suffer from torsional strain and by a rotation around the carbon carbon bond to the staggered conformation around 12.5 kJ/mol of torsional energy is released.
See also
References
- ↑ Eliel, Ernest L.; Wilen, Samuel H. (1994). Stereochemistry of Organic Compounds. Wiley. p. 1197. ISBN 978-0-471-01670-0.