Dodecane
| | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Dodecane[1] | |
| Identifiers | |
| 112-40-3 | |
| 3D model (Jmol) | Interactive image |
| 1697175 | |
| ChEBI | CHEBI:28817 |
| ChEMBL | ChEMBL30959 |
| ChemSpider | 7890 |
| DrugBank | DB02771 |
| ECHA InfoCard | 100.003.607 |
| EC Number | 203-967-9 |
| 201408 | |
| KEGG | C08374 |
| MeSH | n-dodecane |
| PubChem | 8182 |
| RTECS number | JR2125000 |
| UNII | 11A386X1QH |
| |
| |
| Properties | |
| C12H26 | |
| Molar mass | 170.34 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Gasoline-like to odorless |
| Density | 0.7495 g mL−1 at 25 °C[2] |
| Melting point | −10.0 to −9.3 °C; 14.1 to 15.2 °F; 263.2 to 263.8 K |
| Boiling point | 214 to 218 °C; 417 to 424 °F; 487 to 491 K |
| log P | 6.821 |
| Vapor pressure | 18 Pa (at 25 °C)[3] |
| Henry's law constant (kH) |
1.4 nmol Pa−1 kg−1 |
| Refractive index (nD) |
1.421 |
| Viscosity | 1.34 mPa s |
| Thermochemistry | |
| 376.00 J K−1 mol−1 | |
| Std molar entropy (S |
490.66 J K−1 mol−1 |
| Std enthalpy of formation (ΔfH |
−353.5–−350.7 kJ mol−1 |
| Std enthalpy of combustion (ΔcH |
−7901.74 kJ mol−1 |
| Hazards | |
| Safety data sheet | hazard.com |
| GHS pictograms | 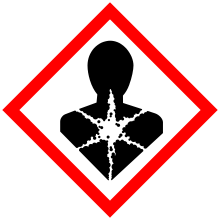 |
| GHS signal word | DANGER |
| H304 | |
| P301+310, P331 | |
| EU classification (DSD) |
|
| R-phrases | R65 |
| S-phrases | S62 |
| NFPA 704 | |
| Flash point | 71 °C (160 °F; 344 K) |
| 205 °C (401 °F; 478 K) | |
| Explosive limits | 0.6% |
| Related compounds | |
| Related alkanes |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Dodecane (also known as dihexyl, bihexyl, adakane 12 or duodecane) is a liquid alkane hydrocarbon with the chemical formula CH3(CH2)10CH3 (or C12H26), an oily liquid of the paraffin series. It has 355 isomers.
It is used as a solvent, distillation chaser, and scintillator component. It is used as a diluent for tributyl phosphate (TBP) in reprocessing plants.[4]
Combustion reaction
The combustion reaction of dodecane is as follows:
- C12H26(l) + 18.5 O2(g) → 12 CO2(g) + 13 H2O(g)
One litre of fuel needs about 15 kg of air to burn, and generates 2.3 kg (or 1.2 m3) of CO2 upon complete combustion.
Jet fuel surrogate
In recent years, n-dodecane has garnered attention as a possible surrogate for kerosene-based fuels such as Jet-A, S-8, and other conventional aviation fuels. It is considered a second-generation fuel surrogate designed to emulate the laminar flame speed, largely supplanting n-decane, primarily due to its higher molecular mass and lower hydrogen to carbon ratio which better reflect the n-alkane content of jet fuels.
See also
References
- ↑ "n-dodecane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. Retrieved 4 January 2012.
- ↑ http://pubchem.ncbi.nlm.nih.gov/compound/dodecane#section=Solubility
- ↑ http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=8182#x400
- ↑ Rydberg, Jan (2004). Solvent Extraction Principles and Practice. Marcel Dekker. p. 524. ISBN 0-8247-5063-2.
External links
- Caudwell, D.R.; Trusler, J.P.M.; Vesovic, V.; Wakeham, W.A. (2003-06-16). "The Viscosity and Density of n-Dodecane and n-Octadecane at Pressures up to 200 mPa and Temperatures up to 473 K" (pdf). NIST. Retrieved 2007-10-09.
- Material Safety Data Sheet for Dodecane
- Dodecane, Dr. Duke's Phytochemical and Ethnobotanical Databases
