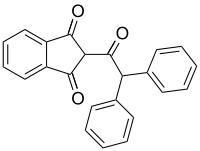
Diphenadione
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-(Diphenylacetyl)-1H-indene-1,3(2H)-dione | |
| Other names
Diphacinone; Diphenandione | |
| Identifiers | |
| 82-66-6 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL1413199 |
| ChemSpider | 6463 |
| ECHA InfoCard | 100.001.304 |
| KEGG | D07136 |
| PubChem | 6719 |
| UNII | 54CA01C6JX |
| |
| |
| Properties | |
| C23H16O3 | |
| Molar mass | 340.38 g·mol−1 |
| Pharmacology | |
| B01AA10 (WHO) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Diphenadione is a vitamin K antagonist that has anticoagulant effects and is used as a rodenticide against rats, mice, voles, ground squirrels and other rodents. The chemical compound is an anti-coagulant with active half-life longer than warfarin and other synthetic indandione anticoagulants.[1]
It is toxic to mammals, in all forms, to all modes of contact, including via skin; exposure and oral ingestion of the toxin may cause irregular heartbeat and major maladies associated with its impact on blood clotting, depending on dose.[2] As a "second-generation" anticoagulant, diphenadione is more toxic than the first generation compounds (e.g., warfarin).[3]:436 For purposes of treating toxicity on exposure, diphenadione is grouped with other vitamin K antagonists (coumarins and indandiones); despite being directed at rodents and being judged as less hazardous to humans and domestic animals than other rodenticides in use (by the U.S. Environmental Protection Agency), indandione anticoagulants, nevertheless, "may cause human toxicity at a much lower dose than conventional 'first-generation anticoagulants'… and can bioaccumulate in the liver."[4]:173
References
- ↑ EXTOXNET Staff (1993-09-01). "Diphacinone". EXTOXNET. Retrieved 2011-12-07.
- ↑ Bell Laboratories, Inc. July, 1990. Diphacinone Technical: MSDS. Bell Labs, Madison, WI.
- ↑ Murphy, Michael J.; Talcott, Patricia A. (2013). "Anticoagulant Rodenticides (Ch. 32)". In Peterson, Michael E.; Talcott, Patricia A. Small Animal Toxicology (3rd ed.). St. Louis, MO, US: Elsevier Health Sciences. pp. 435–446, esp. 435–439. ISBN 0323241980. Retrieved 5 April 2016.
- ↑ Reigart, J. Routt & Roberts, James R. (Eds.) (2013). "Rodenticides (Ch. 18, § Coumarins and Indandiones)" (PDF). Recognition and Management of Pesticide Poisonings (6th ed.). Corvallis, OR, US: National Pesticide Information Center (Oregon State University and the U.S. Environmental Protection Agency. Retrieved 5 April 2016.
The first-generation anticoagulants, for example, are reasonably effective against pest rodents and are less toxic than second-generation anticoagulants… / Very small amounts of the extremely toxic rodenticides sodium fluoroacetate, fluoracetamide, strychnine, crimidine, yellow phosphorus, zinc phosphide and thallium sulfate can cause severe and even fatal poisoning. Cholecalciferol is also a highly toxic agent. The anticoagulants, indandiones and red squill, are less hazardous to humans and domestic animals. Some of the newer anticoagulant compounds, termed 'second-generation anticoagulants,' may cause human toxicity at a much lower dose than conventional 'first-generation anticoagulants'… and can bioaccumulate in the liver…
[p. 173, emphasis in source].
Further reading
- "Diphacinone - toxicity, ecological toxicity and regulatory information". Pesticideinfo.org. Retrieved 2011-12-07. An online pesticide database that gives more information about the safety issues associated with use of diphenadione.
- Reigart, J. Routt & Roberts, James R. (Eds.) (2013). "Rodenticides (Ch. 18, § Coumarins and Indandiones)" (PDF). Recognition and Management of Pesticide Poisonings (6th ed.). Corvallis, OR, US: National Pesticide Information Center (Oregon State University and the U.S. Environmental Protection Agency. pp. 173–187. Retrieved 5 April 2016. A safety handbook that explains how incidents of poisoning by various rodenticides are treated.