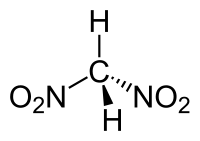
Dinitromethane
 | |
| Identifiers | |
|---|---|
| 625-76-3 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 55118 |
| PubChem | 61172 |
| |
| |
| Properties | |
| CH2N2O4 | |
| Molar mass | 106.04 g·mol−1 |
| Boiling point | 39 to 40 °C (102 to 104 °F; 312 to 313 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Dinitromethane is an organic compound with the chemical formula CH2(NO2)2.[1] Purified dinitromethane is a colorless liquid with a weak pleasant odor. It is relatively stable at room temperature and can be safely stored for months at 0 °C.[2]
Synthesis
The potassium salt of dinitromethanide, KCH(NO2)2, was first prepared by Villiers in 1884 by reduction of bromodinitromethane.[3] Hydrogen fluoride and the potassium salt of dinitromethane react in diethyl ether to form dinitromethane.[2] Free dinitromethane was previously understood to be a pale, yellow oil that decomposed rapidly at ambient temperatures.[4]
Dinitromethane is also produced as a byproduct from the production of the explosive RDX.[5]
Safety
The transportation of dinitromethane is forbidden by the U.S. Department of Transportation.[6]
References
- ↑ Linstrom, P. J.; Mallard, W. G. (eds.). "Dinitromethane". NIST Chemistry WebBook, NIST Standard Reference Database Number 69. NIST.
- 1 2 Legin, G. Ya.; Okhlobystina, L. V.; Fainzilberg, A. A. (1965). "Preparation of pure dinitromethane and its physical properties". Russian Chemical Bulletin. 14 (12): 2190–2191. doi:10.1007/BF00846018.
- ↑ Villiers, R. (1884). "Sur le bromure d'èthylène tétranitré". Bulletin de la Société Chimique de France. 41: 281.
- ↑ Duden, P. (1893). "Ueber das Dinitromethan". Berichte der Deutschen Chemischen Gesellschaft. 26 (3): 3003–3011. doi:10.1002/cber.189302603135.
- ↑ Luo, K.-M., Lin, S.-H., Chang, J.-G., Huang, T.-H. (2002), "Evaluations of kinetic parameters and critical runaway conditions in the reaction system of hexamine-nitric acid to produce RDX in a non-isothermal batch reactor", Journal of Loss Prevention in the Process Industries 15 (2): 119–127,
- ↑ "DOT Hazardous Materials". Retrieved 2012-03-01.