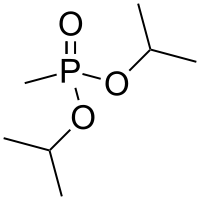
Diisopropyl methylphosphonate
 | |
| Names | |
|---|---|
| IUPAC name
Diisopropyl methylphosphonate [1] | |
| Other names
2-(Methyl-propan-2-yloxyphosphoryl)oxypropane | |
| Identifiers | |
| 1445-75-6 | |
| 3D model (Jmol) | Interactive image |
| Abbreviations | DIMP |
| ChEBI | CHEBI:77325 |
| ChemSpider | 2964 |
| ECHA InfoCard | 100.014.451 |
| PubChem | 3073 |
| |
| |
| Properties | |
| C7H17O3P | |
| Molar mass | 180.18 g·mol−1 |
| Density | 0.976 g/mL |
| Boiling point | 215 °C (419 °F; 488 K) |
| Hazards | |
| Flash point | 98 °C (208 °F; 371 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diisopropyl methylphosphonate (DIMP), also known as diisopropyl methane-phosphonate and phosphonic acid and methyl-bis-(1-methylethyl)ester, is a chemical by-product in the production of sarin gas.[2] Diisopropylmethylphosphonate is a colorless liquid that has been shown to affect the hematological (blood forming) system in animals.[3] Its chemical formula is C7H17O3P.[4]
History
Diisopropyl methylphosphonate is a chemical by-product resulted from the manufacture of sarin (GB), a nerve gas that was produced for military purpose in the 1950s.[5]
Use
No commercial uses of diisopropyl methylphosphonate are known to exist.[6]
Occurrences
Diisopropyl methylphosphonate is not known to occur naturally in the environment.
Productions
Synthesis
Diisopropyl methylphosphonate can be prepared by a gradual addition of triisopropyl phosphite with methyl iodide, utilizing distillation technique.
References
- ↑ Diisopropyl methylphosphonate
- ↑ "ATSDR - Toxic Substances - Diisopropyl Methylphosphonate (DIMP)". Atsdr.cdc.gov. 2011-03-03. Retrieved 2012-10-18.
- ↑ "tf119" (PDF). Retrieved 2012-10-18.
- ↑ "Chemicals & Reagents Of Adeviq-Spin Poland". Chemicals.pl. Retrieved 2012-10-18.
- ↑ ATSDR - Toxic Substances - Diisopropyl Methylphosphonate (DIMP)
- ↑ http://www.atsdr.cdc.gov/toxprofiles/tp119-c4.pdf