Dental porcelain
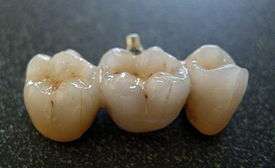
Dental porcelain (also known as dental ceramic) is a porcelain used by a dental technician to create biocompatible lifelike dental restorations, such as crowns, bridges, and veneers, for the patient. Evidence suggests they are effective (they are biocompatible, esthetic, insoluble and have hardness of 7 on the Mohs scale), although for certain dental prostheses (such as three-unit molars porcelain fused to metal or in complete porcelain group) only zirconia-based restorations are recommended.[1]
The word "ceramic" is derived from the Greek word κέραμος keramos, which means "potter's clay".[2] It came from the ancient art of fabricating pottery where mostly clay was fired to form a hard, brittle object. A more modern definition is a material that contains metallic and non-metallic elements (usually oxygen). These materials can be defined by their inherent properties; they form hard, stiff, and brittle materials due to the nature of their inter-atomic bonding, which is ionic and covalent. Contrast that to a metal; metals are non-brittle (display elastic behavior), and ductile (display plastic behavior). This is because of the nature of the inter-atomic bonding, which is called a metallic bond. These bonds are defined by a cloud of shared electrons that can easily move when energy is applied. This is what makes most metals great conductors. Ceramics can be very translucent to very opaque. In general, the more glassy the microstructure (i.e. noncrystalline) the more translucent it will appear, and the more crystalline, the more opaque.[3]
Methods
The dentist will usually specify a shade or combination shades for different parts of the restoration, corresponding to a set of bottles in the lab containing the porcelain powder. A common shade system used is the Vita guide (Vita Classical and Vita 3D Master). There are two types of porcelain restorations: porcelain fused to metal and complete porcelain. For porcelain fused to metal, the black color of metal is first masked with an opaque layer to make it a shade of white and then consecutive layers are built up. The powder corresponding to the desired shade of dentine base is mixed with water, and then fired. Further layers are built up to mimic the natural translucency of the enamel of the tooth. The porcelain is fused to a semi-precious metal or precious metal such as gold, for extra strength. Many systems use an aluminium oxide or zirconium oxide or zirconia core instead of metal that makes complete porcelain restorations.
Recent developments in CAD/CAM dentistry use special partially sintered ceramic (zirconia), glass-bonded ceramic ("Vitablock") or glass-ceramic ("ips.emax" lithium disilicate)[4] formed into machinable blocks, which are fired again after machining. CAD/CAM restorations created with glass-ceramic CEREC technology appear to last well.[5]
Utilizing in-office CAD/CAM technology, clinicians can design, fabricate and place all-ceramic inlays, onlays, crowns and veneers in a single patient visit. The ceramic restorations produced by this method have demonstrated excellent fit, strength and longevity. Two basic techniques can be used for CAD/CAM restorations.
- Chairside single-visit technique
- Integrated chairside–laboratory CAD/CAM procedure.[6]
Classifications
Ceramics can be classified by their microstructure (i.e., amount and type of crystalline phase and glass composition).[7] They can also be classified by the processing technique (power-liquid, pressed, or machined).
Microstructural classifications
At the microstructural level, we can define ceramics by the nature of their composition of amorphous-to-crystalline ratio. There can be infinite variability of the microstructures of materials, but they can be broken down into four basic compositional categories, with a few subgroups:composition category 1 – glass-based systems (mainly silica),composition category 2 – glass-based systems (mainly silica) with fillers, usually crystalline (typically leucite or, more recently, lithium disilicate),composition category 3 – crystalline- based systems with glass fillers (mainly alumina) andcomposition category 4 – polycrystalline solids (alumina and zirconia).
Dental porcelain is generally regarded as biologically inert. However, other toxicities may exist from depleted uranium and some of the other accessory materials, and the fillings may increase wear on opposing teeth.[8]
References
- ↑ Della Bona A, Kelly JR (September 2008). "The clinical success of all-ceramic restorations". J Am Dent Assoc. 139. Suppl: 8S–13S. PMID 18768903.
- ↑ Liddell & Scott, An Intermediate Greek–English Lexicon
- ↑ McLaren, Cao, Edward A, Phong Tran (October 2009). "Ceramics in Dentistry—Part I: Classes of Materials". Inside Dentistry. 5 (9).
- ↑ Tysowsky, George. "The Science Behind Lithium Disilicate". Retrieved Feb 2012. Check date values in:
|access-date=(help) - ↑ Fasbinder DJ (September 2006). "Clinical performance of chairside CAD/CAM restorations". J Am Dent Assoc. 137. Suppl: 22S–31S. PMID 16950934.
- ↑ Shenoy, Shenoy, Arvin, Nina (Oct–Dec 2010). "Dental ceramics: An update". Journal of Conservative Dentistry. 13 (4): 195–203. doi:10.4103/0972-0707.73379. PMC 3010023
 . PMID 21217946.
. PMID 21217946. - ↑ McLaren, Tran, Edward A, CP (October 2009). "Ceramics in Dentistry-Part I: Classes of Materials". Inside Dentistry. 5 (9). Retrieved 29 August 2013.
- ↑ Mackert JR (September 1992). "Side-effects of dental ceramics". Adv. Dent. Res. 6: 90–3. PMID 1337968.