Cyanobacteria
| Cyanobacteria Temporal range: 3500–0Ma | |
|---|---|
| | |
| Tolypothrix sp. | |
| Scientific classification | |
| Domain: | Bacteria |
| Kingdom: | Eubacteria |
| Phylum: | Cyanobacteria Stanier, 1973 |
| Orders[1] | |
| Synonyms | |
| |
Cyanobacteria /saɪˌænoʊbækˈtɪəriə/, also known as Cyanophyta, is a phylum of bacteria that obtain their energy through photosynthesis.[4] The name "cyanobacteria" comes from the color of the bacteria (Greek: κυανός (kyanós) = blue). Sometimes, they are called blue-green algae, and incorrectly so, because cyanobacteria are prokaryotes and the term "algae" is reserved for eukaryotes.[5]
Like other prokaryotes, cyanobacteria have no membrane-sheathed organelles. Photosynthesis is performed in distinctive folds in the outer membrane of the cell (unlike green plants which use organelles adapted for this specific role, called chloroplasts). Biologists commonly agree that chloroplasts found in eukaryotes, have their ancestry in cyanobacteria, via a process called endosymbiosis.
By producing oxygen as a byproduct of photosynthesis, cyanobacteria are thought to have converted the early oxygen-poor, reducing atmosphere, into an oxidizing one, causing the "rusting of the Earth"[6] and the Great Oxygenation Event, that dramatically changed the composition of life forms and led to the near-extinction of anaerobic organisms.
Description
Cyanobacteria are a group of photosynthetic, nitrogen fixing bacteria that live in a wide variety of habitats such as moist soils and in water. They may be free-living or form symbiotic relationships with plants or with lichen-forming fungi as in the lichen genus Peltigera.[7] They range from unicellular to filamentous and include colonial species. Colonies may form filaments, sheets, or even hollow balls. Some filamentous species can differentiate into several different cell types: vegetative cells, the normal, photosynthetic cells that are formed under favorable growing conditions; akinetes, climate-resistant spores that may form when environmental conditions become harsh; and thick-walled heterocysts, which contain the enzyme nitrogenase, vital for nitrogen fixation.
Nitrogen fixation
Cyanobacteria can fix atmospheric nitrogen in anaerobic conditions by means of specialized cells called heterocysts. Heterocysts may also form under the appropriate environmental conditions (anoxic) when fixed nitrogen is scarce. Heterocyst-forming species are specialized for nitrogen fixation and are able to fix nitrogen gas into ammonia (NH3), nitrites (NO−
2) or nitrates (NO−
3), which can be absorbed by plants and converted to protein and nucleic acids (atmospheric nitrogen is not bioavailable to plants, except for those having endosymbiotic nitrogen-fixing bacteria, especially the Fabaceae family, among others). Most importantly they are unicellular.
Free-living cyanobacteria are present in the water column in rice paddies, and cyanobacteria can be found growing as epiphytes on the surfaces of the green alga, Chara, where they may fix nitrogen.[8] Cyanobacteria such as (Anabaena, a symbiont of the aquatic fern Azolla), can provide rice plantations with biofertilizer.[9]
Morphology

Many cyanobacteria form motile filaments of cells, called hormogonia, that travel away from the main biomass to bud and form new colonies elsewhere. The cells in a hormogonium are often thinner than in the vegetative state, and the cells on either end of the motile chain may be tapered. To break away from the parent colony, a hormogonium often must tear apart a weaker cell in a filament, called a necridium.
Each individual cell of a cyanobacterium typically has a thick, gelatinous cell wall. They lack flagella, but hormogonia of some species can move about by gliding along surfaces. Many of the multicellular filamentous forms of Oscillatoria are capable of a waving motion; the filament oscillates back and forth. In water columns, some cyanobacteria float by forming gas vesicles, as in archaea. These vesicles are not organelles as such. They are not bounded by lipid membranes, but by a protein sheath.
Ecology
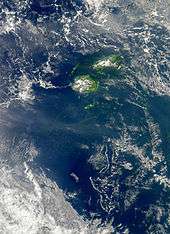
Cyanobacteria can be found in almost every terrestrial and aquatic habitat—oceans, fresh water, damp soil, temporarily moistened rocks in deserts, bare rock and soil, and even Antarctic rocks. They can occur as planktonic cells or form phototrophic biofilms. They are found in almost every endolithic ecosystem.[10] A few are endosymbionts in lichens, plants, various protists, or sponges and provide energy for the host. Some live in the fur of sloths, providing a form of camouflage.[11]
Aquatic cyanobacteria are known for their extensive and highly visible blooms that can form in both freshwater and marine environments. The blooms can have the appearance of blue-green paint or scum. These blooms can be toxic, and frequently lead to the closure of recreational waters when spotted. Marine bacteriophages are significant parasites of unicellular marine cyanobacteria.[12]
Cyanobacteria prefer calm waters, such as those provided by ponds and lakes. Their life cycles are disrupted when the water naturally or artificially mixes from churning currents caused by the flowing water of streams or the churning water of fountains. For this reason blooms of cyanobacteria seldom occur in rivers unless the water is flowing slowly. When the bacteria are found in rivers, they have usually come from the outfall of lakes upstream from the sampling point.
Cyanobacteria are a growing concern for drinking water utilities who use lakes and rivers as their source water. The bacteria can interfere with treatment in various ways, primarily by plugging filters (often large beds of sand and similar media), and by producing cyanotoxins, which have the potential of causing serious illness if consumed.
Some of these organisms contribute significantly to global ecology and the oxygen cycle. The tiny marine cyanobacterium Prochlorococcus was discovered in 1986 and accounts for more than half of the photosynthesis of the open ocean.[13] Many cyanobacteria even display the circadian rhythms that were once thought to exist only in eukaryotic cells (see bacterial circadian rhythms).
"Cyanobacteria are arguably the most successful group of microorganisms on earth. They are the most genetically diverse; they occupy a broad range of habitats across all latitudes, widespread in freshwater, marine, and terrestrial ecosystems, and they are found in the most extreme niches such as hot springs, salt works, and hypersaline bays. Photoautotrophic, oxygen-producing cyanobacteria created the conditions in the planet's early atmosphere that directed the evolution of aerobic metabolism and eukaryotic photosynthesis. Cyanobacteria fulfill vital ecological functions in the world's oceans, being important contributors to global carbon and nitrogen budgets." – Stewart and Falconer[14]
Photosynthesis
While contemporary cyanobacteria are linked to the plant kingdom as descendants of the endosymbiotic progenitor of the chloroplast, there are several features which are unique to this group.
Carbon fixation
Cyanobacteria use the energy of sunlight to drive photosynthesis, a process where the energy of light is used to split water molecules into oxygen, protons, and electrons. Because they are aquatic organisms, they typically employ several strategies which are collectively known as a "carbon concentrating mechanism" to aid in the acquisition of inorganic carbon (CO2 or bicarbonate). Among the more specific strategies is the widespread prevalence of the bacterial microcompartments known as carboxysomes.[15] These icosahedral structures are composed of hexameric shell proteins that assemble into cage-like structures that can be several hundreds of nanometers in diameter. It is believed that these structures tether the CO2-fixing enzyme, RuBisCO, to the interior of the shell, as well as the enzyme carbonic anhydrase, using the paradigm of metabolic channeling to enhance the local CO2 concentrations and thus increase the efficiency of the RuBisCO enzyme.[16]
Electron transport
In contrast to chloroplast-containing eukaryotes, cyanobacteria lack compartmentalization of their thylakoid membranes, which are contiguous with the plasma membrane. Thus, the protein complexes involved in respiratory energy metabolism share several mobile energy carrier pools (e.g., the Quinone pool, cytochrome c, ferredoxins), so photosynthetic and respiratory metabolism interact with each other. Furthermore, there is a tremendous diversity among the respiratory components between species. Thus cyanobacteria can be said to have a "branched electron transport chain", analogous to the situation in purple bacteria.
While most of the high-energy electrons derived from water are used by the cyanobacterial cells for their own needs, a fraction of these electrons may be donated to the external environment via electrogenic activity.[17]
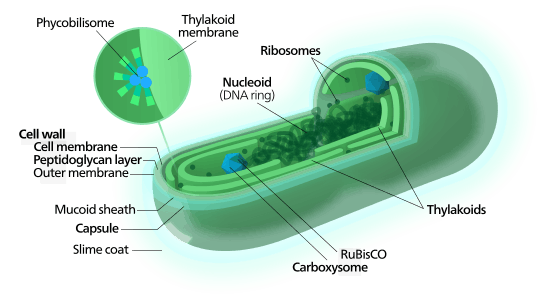
Metabolism and organelles
As prokaryotes, cyanobacteria do not have nuclei or an internal membrane system. In most forms, the photosynthetic machinery is embedded into folds of the external cell membrane, called thylakoids. Cyanobacteria get their colour from the bluish pigment phycocyanin, which they use to capture light for photosynthesis. In general, photosynthesis in cyanobacteria uses water as an electron donor and produces oxygen as a byproduct, though some may also use hydrogen sulfide[18] a process which occurs among other photosynthetic bacteria such as the purple sulfur bacteria. Carbon dioxide is reduced to form carbohydrates via the Calvin cycle.The large amounts of oxygen in the atmosphere are considered to have been first created by the activities of ancient cyanobacteria. They are often found as symbionts with a number of other groups of organisms such as fungi (lichens), corals, pteridophytes (Azolla), angiosperms (Gunnera), etc.
Many cyanobacteria are able to reduce nitrogen and carbon dioxide under aerobic conditions, a fact that may be responsible for their evolutionary and ecological success. The water-oxidizing photosynthesis is accomplished by coupling the activity of photosystem (PS) II and I (Z-scheme). In anaerobic conditions, they are able to use only PS I—cyclic photophosphorylation—with electron donors other than water (hydrogen sulfide, thiosulphate, or even molecular hydrogen[19]) just like purple photosynthetic bacteria. Furthermore, they share an archaeal property, the ability to reduce elemental sulfur by anaerobic respiration in the dark. Their photosynthetic electron transport shares the same compartment as the components of respiratory electron transport. Their plasma membrane contains only components of the respiratory chain, while the thylakoid membrane hosts an interlinked respiratory and photosynthetic electron transport chain. The terminal oxidases in the thylakoid membrane respiratory/photosynthetic electron transport chain are essential for survival to rapid light changes, although not for dark maintenance under conditions where cells are not light stressed.[20]
Attached to the thylakoid membrane, phycobilisomes act as light-harvesting antennae for the photosystems.[21] The phycobilisome components (phycobiliproteins) are responsible for the blue-green pigmentation of most cyanobacteria. The variations on this theme are due mainly to carotenoids and phycoerythrins that give the cells their red-brownish coloration. In some cyanobacteria, the color of light influences the composition of phycobilisomes. In green light, the cells accumulate more phycoerythrin, whereas in red light they produce more phycocyanin. Thus, the bacteria appear green in red light and red in green light. This process of complementary chromatic adaptation is a way for the cells to maximize the use of available light for photosynthesis.
A few genera lack phycobilisomes and have chlorophyll b instead (Prochloron, Prochlorococcus, Prochlorothrix). These were originally grouped together as the prochlorophytes or chloroxybacteria, but appear to have developed in several different lines of cyanobacteria. For this reason, they are now considered as part of the cyanobacterial group.
There are some groups capable of heterotrophic growth,[22] while others are parasitic, causing diseases in invertebrates or eukaryotic algae (e.g., the black band disease).[23][24][25]
Relationship to chloroplasts
|
and similar) and basal cyanobacteria[26]
Chloroplasts found in eukaryotes (algae and plants) appear to have evolved from an endosymbiotic relation with cyanobacteria.[27][28] This endosymbiotic theory is supported by various structural and genetic similarities.[29] Primary chloroplasts are found among the "true plants" or green plants – species ranging from sea lettuce to evergreens and flowers that contain chlorophyll b – as well as among the red algae and glaucophytes, marine species that contain phycobilins. It now appears that these chloroplasts probably had a single origin, in an ancestor of the clade called Archaeplastida. However this does not necessitate origin from cyanobacteria themselves; microbiology is still undergoing profound classification changes and entire domains (such as Archaea) are poorly mapped and understood. Other algae likely took their chloroplasts from these forms by secondary endosymbiosis or ingestion.
Classification
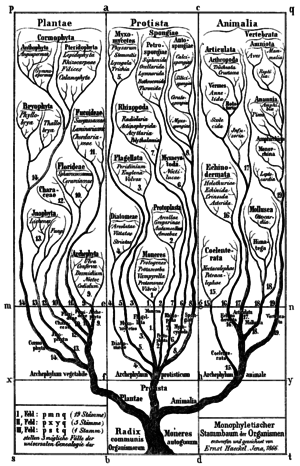
Historically, bacteria were first classified as plants constituting the class Schizomycetes, which along with the Schizophyceae (blue-green algae/Cyanobacteria) formed the phylum Schizophyta,[30] then in the phylum Monera in the kingdom Protista by Haeckel in 1866, comprising Protogens, Protamaeba, Vampyrella, Protomonae, and Vibrio, but not Nostoc and other cyanobacteria, which were classified with algae,[31] later reclassified as the Prokaryotes by Chatton.[32]
The cyanobacteria were traditionally classified by morphology into five sections, referred to by the numerals I-V. The first three – Chroococcales, Pleurocapsales, and Oscillatoriales – are not supported by phylogenetic studies. The latter two – Nostocales and Stigonematales – are monophyletic, and make up the heterocystous cyanobacteria.
The members of Chroococales are unicellular and usually aggregate in colonies. The classic taxonomic criterion has been the cell morphology and the plane of cell division. In Pleurocapsales, the cells have the ability to form internal spores (baeocytes). The rest of the sections include filamentous species. In Oscillatoriales, the cells are uniseriately arranged and do not form specialized cells (akinetes and heterocysts). In Nostocales and Stigonematales, the cells have the ability to develop heterocysts in certain conditions. Stigonematales, unlike Nostocales, include species with truly branched trichomes.
Most taxa included in the phylum or division Cyanobacteria have not yet been validly published under the Bacteriological Code, except:
- The classes Chroobacteria, Hormogoneae, and Gloeobacteria
- The orders Chroococcales, Gloeobacterales, Nostocales, Oscillatoriales, Pleurocapsales, and Stigonematales
- The families Prochloraceae and Prochlorotrichaceae
- The genera Halospirulina, Planktothricoides, Prochlorococcus, Prochloron, and Prochlorothrix
The remainder are validly published under the International Code of Nomenclature for algae, fungi, and plants.
Formerly, some bacteria, like Beggiatoa, were thought to be colorless Cyanobacteria.[33]
Earth history
Stromatolites are layered biochemical accretionary structures formed in shallow water by the trapping, binding, and cementation of sedimentary grains by biofilms (microbial mats) of microorganisms, especially cyanobacteria.[34]

During the Precambrian, stromatolite communities of microorganisms grew in most marine and non-marine environments in the photic zone. After the Cambrian explosion of marine animals, grazing on the stromatolite mats by herbivores greatly reduced the occurrence of the stromatolites in marine environments. Since then, they are found mostly in hypersaline conditions where grazing invertebrates cannot live (e.g. Shark Bay, Western Australia). Stromatolites provide ancient records of life on Earth by fossil remains which might date from more than 3.5 Ga ago, but this is disputed.[35] As of 2010 the oldest undisputed evidence of cyanobacteria is from 2.1 Ga ago, but there is some evidence for them as far back as 2.7 Ga ago. Oxygen levels in the atmosphere remained around or below 1% of today's level until 2.4 Ga ago (the Great Oxygenation Event). The rise in oxygen may have caused a fall in methane levels, and triggered the Huronian glaciation from around 2.4 to 2.1 Ga ago. In this way, cyanobacteria may have killed off much of the other bacteria of the time.[36]
Oncolites are sedimentary structures composed of oncoids, which are layered structures formed by cyanobacterial growth. Oncolites are similar to stromatolites, but instead of forming columns, they form approximately spherical structures that were not attached to the underlying substrate as they formed.[37] The oncoids often form around a central nucleus, such as a shell fragment,[38] and a calcium carbonate structure is deposited by encrusting microbes. Oncolites are indicators of warm waters in the photic zone, but are also known in contemporary freshwater environments.[39] These structures rarely exceed 10 cm in diameter.
Biotechnology and applications
The unicellular cyanobacterium Synechocystis sp. PCC6803 was the third prokaryote and first photosynthetic organism whose genome was completely sequenced.[40] It continues to be an important model organism.[41] Cyanothece ATCC 51142 is an important diazotrophic model organism. The smallest genomes have been found in Prochlorococcus spp. (1.7 Mb)[42][43] and the largest in Nostoc punctiforme (9 Mb).[44] Those of Calothrix spp. are estimated at 12–15 Mb,[45] as large as yeast.

Recent research has suggested the potential application of cyanobacteria to the generation of renewable energy by converting sunlight into electricity. Internal photosynthetic pathways can be coupled to chemical mediators that transfer electrons to external electrodes.[46] Currently, efforts are underway to commercialize algae-based fuels such as diesel, gasoline, and jet fuel.[17][47][48]

Researchers from a company called Algenol have cultured genetically modified cyanobacteria in sea water inside a clear plastic enclosure so they first make sugar (pyruvate) from CO2 and the water via photosynthesis. Then, the bacteria secrete ethanol from the cell into the salt water. As the day progresses, and the solar radiation intensifies, ethanol concentrations build up and the ethanol itself evaporates onto the roof of the enclosure. As the sun recedes, evaporated ethanol and water condense into droplets, which run along the plastic walls and into ethanol collectors, from where it is extracted from the enclosure with the water and ethanol separated outside the enclosure. As of March 2013, Algenol was claiming to have tested its technology in Florida and to have achieved yields of 9,000 US gallons per acre per year.[49] This could potentially meet US demands for ethanol in gasoline in 2025, assuming a B30 blend, from an area of around half the size of California’s San Bernardino County, requiring less than one-tenth of the area than ethanol from other biomass, such as corn, and only very limited amounts of fresh water.[50]
Cyanobacteria may possess the ability to produce substances that could one day serve as anti-inflammatory agents and combat bacterial infections in humans.[51]
Spirulina's extracted blue color is used as a natural food coloring in gum and candy.[52]
Researchers from several space agencies argue that cyanobacteria could be used for producing goods for human consumption (food, oxygen...) in future manned outposts on Mars, by transforming materials available on this planet.[53]
Health risks
Cyanobacteria can produce neurotoxins, cytotoxins, endotoxins, and hepatotoxins (i.e. the microcystin-producing bacteria species microcystis), and are called cyanotoxins.
Specific toxins include, anatoxin-a, anatoxin-as, aplysiatoxin, cyanopeptolin, cylindrospermopsin, domoic acid, nodularin R (from Nodularia), neosaxitoxin, and saxitoxin. Cyanobacteria reproduce explosively under certain conditions. This results in algal blooms, which can become harmful to other species, and pose a danger to humans and animals, if the cyanobacteria involved produce toxins. Several cases of human poisoning have been documented, but a lack of knowledge prevents an accurate assessment of the risks.[54][55][56]
Recent studies suggest that significant exposure to high levels of cyanobacteria producing toxins such as BMAA can cause amyotrophic lateral sclerosis (ALS). People living within a half-mile of cyanobacterially contaminated lakes have had a 2.3-times greater risk of developing ALS than the rest of the population; people around New Hampshire’s Lake Mascoma had an up to 25 times greater risk of ALS than the expected incidence.[57] BMAA from desert crusts found throughout Qatar might have contributed to higher rates of ALS in Gulf War veterans.[55][58]
Chemical control
Several chemicals can eliminate blue-green algal blooms from water-based systems. They include: calcium hypochlorite, copper sulphate, cupricide, and simazine.[59] The calcium hypochlorite amount needed varies depending on the cyanobacteria bloom, and treatment is needed periodically. According to the Department of Agriculture Australia, a rate of 12 g of 70% material in 1000 l of water is often effective to treat a bloom.[59] Copper sulfate is also used commonly, but no longer recommended by the Australian Department of Agriculture, as it kills livestock, crustaceans, and fish.[59] Culpricide is a chelated copper product that eliminates blooms with lower toxicity risks than copper sulfate. Dosage recommendations vary from 190 ml to 4.8 l per 1000 m2.[59] Ferric alum treatments at the rate of 50 mg/l will reduce algae blooms.[59][60] Simazine, which is also a herbicide, will continue to kill blooms for several days after an application. Simazine is marketed at different strengths (25, 50, and 90%), the recommended amount needed for one cubic meter of water per product is 25% product 8 ml; 50% product 4 ml; or 90% product 2.2 ml.[59]
Dietary supplementation

Some cyanobacteria are sold as food, notably Aphanizomenon flos-aquae and Arthrospira platensis (Spirulina).[61]
Despite the associated toxins which many of the members of this phylum produce, some microalgae also contain substances of high biological value, such as polyunsaturated fatty acids, amino acids (proteins), pigments, antioxidants, vitamins, and minerals.[62] Edible blue-green algae reduce the production of pro-inflammatory cytokines by inhibiting NF-κB pathway in macrophages and splenocytes.[63] Sulfate polysaccharides exhibit immunomodulatory, antitumor, antithrombotic, anticoagulant, anti-mutagenic, anti-inflammatory, antimicrobial, and even antiviral activity against HIV, herpes, and hepatitis.[64]
See also
References
- ↑ Komárek J, Kaštovský J, Mareš J, Johansen JR (2014). "Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach" (PDF). Preslia. 86: 295–335.
- ↑ "Cyanophyceae". Cyanophyceae. Access Science. Retrieved 21 April 2011.
- ↑ Ahoren Oren (2004). "A proposal for further integration of the cyanobacteria under the Bacteriological Code". Int. J. Syst. Evol. Microbiol. 54 (Pt 5): 1895–1902. doi:10.1099/ijs.0.03008-0. PMID 15388760.
- ↑ "Life History and Ecology of Cyanobacteria". University of California Museum of Paleontology. Retrieved 17 July 2012.
- ↑ Allaby, M ed. (1992). "Algae". The Concise Dictionary of Botany. Oxford: Oxford University Press.
- ↑ Schopf, J. W. (2012) "The fossil record of cyanobacteria", pp. 15–36 in Brian A. Whitton (Ed.) Ecology of Cyanobacteria II: Their Diversity in Space and Time. ISBN 9789400738553.
- ↑ Dodds, W.K.; Gudder, D.A.; Mollenhauer, D. (1995). "The ecology of Nostoc". Journal of Phycology. 31: 2–18. doi:10.1111/j.0022-3646.1995.00002.x.
- ↑ Sims, G. K.; Dunigan, E. P. (1984). "Diurnal and seasonal variations in nitrogenase activity (C2H2 reduction) of rice roots". Soil Biology and Biochemistry. 16: 15–18. doi:10.1016/0038-0717(84)90118-4.
- ↑ "Azolla-Anabaena as a Biofertilizer for Rice Paddy Fields in the Po Valley, a Temperate Rice Area in Northern Italy". Azolla-Anabaena as a Biofertilizer for Rice Paddy Fields in the Po Valley, a Temperate Rice Area in Northern Italy. International Journal of Agronomy. Retrieved 21 April 2011.
- ↑ de los Ríos, A; Grube, M; Sancho, LG; Ascaso, C (February 2007). "Ultrastructural and genetic characteristics of endolithic cyanobacterial biofilms colonizing Antarctic granite rocks.". FEMS Microbiology Ecology. 59 (2): 386–95. doi:10.1111/j.1574-6941.2006.00256.x. PMID 17328119.
- ↑ Vaughan, Terry (2011). Mammalogy. Jones and Barlett. p. 21. ISBN 9780763762995.
- ↑ Schultz, Nora (30 August 2009) "Photosynthetic viruses keep world's oxygen levels up". New Scientist.
- ↑ Nadis, Steve (November 2003). "The Cells That Rule the Seas" (PDF). Scientific American. 289 (6): 52–3. doi:10.1038/scientificamerican1203-52. PMID 14631732.
- ↑ Stewart I and Falconer IR (2008) "Cyanobacteria and cyanobacterial toxins" Pages 271–296 in Oceans and human health: risks and remedies from the seas, Eds: Walsh PJ, Smith SL, and Fleming LE. Academic Press, ISBN 0-12-372584-4.
- ↑ Kerfeld, Cheryl A.; Heinhorst, Sabine; Cannon, Gordon C. (2010). "Bacterial Microcompartments". Annual Review of Microbiology. 64 (1): 391–408. doi:10.1146/annurev.micro.112408.134211. ISSN 0066-4227.
- ↑ Long, B. M.; Badger, M. R.; Whitney, S. M.; Price, G. D. (2007). "Analysis of Carboxysomes from Synechococcus PCC7942 Reveals Multiple RuBisCO Complexes with Carboxysomal Proteins CcmM and CcaA". Journal of Biological Chemistry. 282 (40): 29323–29335. doi:10.1074/jbc.M703896200. ISSN 0021-9258. PMID 17675289.
- 1 2 Pisciotta JM, Zou Y, Baskakov IV; Zou; Baskakov (2010). Yang, Ching-Hong, ed. "Light-Dependent Electrogenic Activity of Cyanobacteria". PLoS ONE. 5 (5): e10821. Bibcode:2010PLoSO...510821P. doi:10.1371/journal.pone.0010821. PMC 2876029
 . PMID 20520829.
. PMID 20520829. - ↑ Cohen Y, Jørgensen BB, Revsbech NP, Poplawski R; Jørgensen; Revsbech; Poplawski (1986). "Adaptation to hydrogen sulfide of oxygenic and anoxygenic photosynthesis among Cyanobacteria". Appl. Environ. Microbiol. 51 (2): 398–407. PMC 238881
 . PMID 16346996.
. PMID 16346996. - ↑ Champion Hydrogen-Producing Microbe, ScienceDaily, 15 December 2010
- ↑ Lea-Smith, D. J.; Ross, N.; Zori, M.; Bendall, D. S.; Dennis, J. S.; Scott, S. A.; Smith, A. G.; Howe, C. J. (5 March 2013). "Thylakoid Terminal Oxidases Are Essential for the Cyanobacterium Synechocystis sp. PCC 6803 to Survive Rapidly Changing Light Intensities". Plant Physiology. 162 (1): 484–495. doi:10.1104/pp.112.210260. PMC 3641225
 . PMID 23463783.
. PMID 23463783. - ↑ Grossman, A. R.; Schaefer, M. R.; Chiang, G. G.; Collier, J. L. (1993). "The phycobilisome, a light-harvesting complex responsive to environmental conditions" (PDF). Microbiol. Rev. 57 (3): 725–749. PMC 372933
 .
. - ↑ Smith A.J. (1973). Synthesis of metabolic intermediates. In: Carr N.G. & Whitton B.A. (Eds) The biology of blue-green algae. 676 pp., p. 30.
- ↑ Jangoux, M (1987). "Diseases of Echinodermata. I. Agents microorganisms and protistans" (PDF). Dis. Aquat. Org. 2: 147–162. doi:10.3354/dao002147.
- ↑ Kinne, O. (ed.). Diseases of Marine Animals. Vol. 1. John Wiley & Sons, Chichester, UK.
- ↑ Kristiansen, Aase (1964). "Sarcinastrum urosporae, a Colourless Parasitic Blue-green Alga" (PDF). Phycologia. 4 (1): 19–22. doi:10.2216/i0031-8884-4-1-19.1. Archived from the original (PDF) on 6 January 2015.
- ↑ Enrique Flores AH (2008). The Cyanobacteria: Molecular Biology, Genomics and Evolution. Horizon. p. 3. ISBN 1-904455-15-8.
- ↑ Deusch, O.; et al. (2008). "Genes of cyanobacterial origin in plant nuclear genomes point to a heterocyst-forming plastid ancestor". Mol. Biol. Evol. 25 (4): 748–761. doi:10.1093/molbev/msn022.
- ↑ Ochoa de Alda, JAG; Esteban, R; Diago, ML; Houmard, J (2014). "The plastid ancestor originated among one of the major cyanobacterial lineages". Nature Communications. 5: 4937. doi:10.1038/ncomms5937.
- ↑ Summarised in Cavalier-Smith, T. (2000). "Membrane heredity and early chloroplast evolution". Trends in Plant Science. 5 (4): 174–82. doi:10.1016/S1360-1385(00)01598-3. PMID 10740299.
- ↑ Von Nägeli, C. (1857). Caspary, R., ed. "Bericht über die Verhandlungen der 33. Versammlung deutscher Naturforscher und Aerzte, gehalten in Bonn von 18 bis 24 September 1857" [Report on the negotiations on 33 Meeting of German Natural Scientists and Physicians, held in Bonn, 18 to 24 September 1857]. Botanische Zeitung. 15: 749–776.
- ↑ Haeckel, Ernst (1867). Generelle Morphologie der Organismen. Reimer, Berlin.
- ↑ Chatton, É. (1925). "Pansporella perplexa. Réflexions sur la biologie et la phylogénie des protozoaires". Ann. Sci. Nat. Zool. 10-VII: 1–84.
- ↑ Pringsheim, E.G. (1963). Farblose Algen. Ein beitrag zur Evolutionsforschung. Gustav Fischer Verlag, Stuttgart.
- ↑ Riding, R. (2007). "The term stromatolite: towards an essential definition". Lethaia. 32 (4): 321–330. doi:10.1111/j.1502-3931.1999.tb00550.x.
- ↑ Garwood, Russell J. (2012). "Patterns In Palaeontology: The first 3 billion years of evolution". Palaeontology Online. 2 (11): 1–14. Retrieved June 25, 2015.
- ↑ Lane, Nick (6 February 2010) "First breath: Earth's billion-year struggle for oxygen". New Scientist, pp. 36–9. See accompanying graph as well.
- ↑ Corsetti, F.A.; Awramik, S.M.; Pierce, D.; Pierce, David (2003-04-15). "A complex microbiota from snowball Earth times: Microfossils from the Neoproterozoic Kingston Peak Formation, Death Valley, USA". Proceedings of the National Academy of Sciences. 100 (8): 4399–4404. Bibcode:2003PNAS..100.4399C. doi:10.1073/pnas.0730560100. PMC 153566
 . PMID 12682298.
. PMID 12682298. - ↑ Gutschick, R.C.; Perry, T.G. (1959-11-01). "Sappington (Kinderhookian) sponges and their environment [Montana]". Journal of Paleontology. 33 (6): 977–985. Retrieved 2007-06-28.
- ↑ Riding, Robert. (1991). Calcareous Algae and Stromatolites, p. 32. Springer-Verlag Press.
- ↑ Kaneko, T; Sato, S; Kotani, H; Tanaka, A; Asamizu, E; Nakamura, Y; Miyajima, N; Hirosawa, M; Sugiura, M; Sasamoto, S; Kimura, T; Hosouchi, T; Matsuno, A; Muraki, A; Nakazaki, N; Naruo, K; Okumura, S; Shimpo, S; Takeuchi, C; Wada, T; Watanabe, A; Yamada, M; Yasuda, M; Tabata, S (1996). "Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. Strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions". DNA research : an international journal for rapid publication of reports on genes and genomes. 3 (3): 109–36. doi:10.1093/dnares/3.3.109. PMID 8905231.
- ↑ Tabei Y, Okada K, Tsuzuki M; Okada; Tsuzuki (2007). "Sll1330 controls the expression of glycolytic genes in Synechocystis sp. PCC 6803". Biochem. Biophys. Res. Commun. 355 (4): 1045–50. doi:10.1016/j.bbrc.2007.02.065. PMID 17331473.
- ↑ Rocap, G.; Larimer, F. W.; Lamerdin, J.; Malfatti, S.; Chain, P.; Ahlgren, N. A.; Arellano, A.; Coleman, M.; Hauser, L.; Hess, W. R.; Johnson, Z. I.; Land, M.; Lindell, D.; Post, A. F.; Regala, W.; Shah, M.; Shaw, S. L.; Steglich, C.; Sullivan, M. B.; Ting, C. S.; Tolonen, A.; Webb, E. A.; Zinser, E. R.; Chisholm, S. W. (2003). "Genome divergence in two Prochlorococcus ecotypes reflects oceanic niche differentiation". Nature. 424 (6952): 1042–7. Bibcode:2003Natur.424.1042R. doi:10.1038/nature01947. PMID 12917642.
- ↑ Dufresne, A.; Salanoubat, M.; Partensky, F.; Artiguenave, F.; Axmann, I. M.; Barbe, V.; Duprat, S.; Galperin, M. Y.; Koonin, E. V.; Le Gall, F.; Makarova, K. S.; Ostrowski, M.; Oztas, S.; Robert, C.; Rogozin, I. B.; Scanlan, D. J.; De Marsac, N. T.; Weissenbach, J.; Wincker, P.; Wolf, Y. I.; Hess, W. R. (2003). "Genome sequence of the cyanobacterium Prochlorococcus marinus SS120, a nearly minimal oxyphototrophic genome". Proceedings of the National Academy of Sciences. 100 (17): 10020–5. Bibcode:2003PNAS..10010020D. doi:10.1073/pnas.1733211100. PMC 187748
 . PMID 12917486.
. PMID 12917486. - ↑ Meeks, J. C.; Elhai, J; Thiel, T; Potts, M; Larimer, F; Lamerdin, J; Predki, P; Atlas, R (2001). "An overview of the genome of Nostoc punctiforme, a multicellular, symbiotic cyanobacterium". Photosynthesis Research. 70 (1): 85–106. doi:10.1023/A:1013840025518. PMID 16228364.
- ↑ Herdman, M.; Janvier, M.; Rippka, R.; Stanier, R. Y. (1979). "Genome Size of Cyanobacteria". Journal of General Microbiology. 111: 73–85. doi:10.1099/00221287-111-1-73.
- ↑ Quintana, N.; Van der Kooy, F.; Van de Rhee, M.D.; Voshol, G.P.; Verpoorte, R. (2011). "Renewable energy from Cyanobacteria: energy production optimization by metabolic pathway engineering". Appl Microbiol Biotechnol. 91 (3): 471–490. doi:10.1007/s00253-011-3394-0. PMC 3136707
 . PMID 21691792.
. PMID 21691792. - ↑ Blue green bacteria may help generate 'green' electricity, The Hindu, 21 June 2010
- ↑ Joule wins key patent for GMO cyanobacteria that create fuels from sunlight, CO2, and water. Biofuels Digest (2010-09-14). Retrieved on 2011-04-06.
- ↑ "Algenol Biofuels exceeds 9,000 gallons of ethanol per year per" (PDF). Algenol Biofuels. 6 March 2013.
- ↑ Take it to the Limit: Algenol and rising yields in advanced biofuels. Biofuels Digest (2012-09-25). Retrieved on 2012-09-25.
- ↑ "Nuisance seaweed found to produce compounds with biomedical potential". Sciencedaily.com. 24 May 2012. doi:10.1016/j.chembiol.2012.03.014. Retrieved 1 June 2012.
- ↑ "13 August 2013 Federal Register; FDA Approves Natural Blue Color Additive Extracted from Spirulina".
- ↑ Verseux, Cyprien; Baqué, Mickael; Lehto, Kirsi; de Vera, Jean-Pierre P.; Rothschild, Lynn J.; Billi, Daniela (2016). "Sustainable life support on Mars – the potential roles of cyanobacteria". International Journal of Astrobiology. 15 (1): 65–92. doi:10.1017/S147355041500021X.
- ↑ Thébault, L; Lesne, J; Boutin, J. P. (1995). "Cyanobacteria, their toxins and health risks". Medecine tropicale : revue du Corps de sante colonial. 55 (4): 375–80. PMID 8830224.
- 1 2 Blue-Green Algae (Cyanobacteria) and their Toxins. Hc-sc.gc.ca (2013-01-30). Retrieved on 2014-04-19.
- ↑ Harmful Bloom in Lake Atitlán, Guatemala from NASA Earth Observatory, retrieved on 9 January 2010.
- ↑ Caller, Tracie A.; Doolin, James W.; Haney, James F.; Murby, Amanda J.; West, Katherine G.; Farrar, Hannah E.; Ball, Andrea; Harris, Brent T.; Stommel, Elijah W. (2009). "A cluster of amyotrophic lateral sclerosis in New Hampshire: A possible role for toxic cyanobacteria blooms". Amyotrophic Lateral Sclerosis. 10: 101–8. doi:10.3109/17482960903278485. PMID 19929741.
- ↑ Cox, Paul Alan; Richer, Renee; Metcalf, James S.; Banack, Sandra Anne; Codd, Geoffrey A.; Bradley, Walter G. (2009). "Cyanobacteria and BMAA exposure from desert dust: A possible link to sporadic ALS among Gulf War veterans". Amyotrophic Lateral Sclerosis. 10: 109–17. doi:10.3109/17482960903286066. PMID 19929742.
- 1 2 3 4 5 6 Main, D.C. (2006). "Toxic Algae Blooms" (PDF). Veterinary Pathologist, South Perth. agric.wa.gov.au. Retrieved 18 November 2014.
- ↑ May, V., & Baker, H. (1978). "Reduction of toxic algae in farm dams by ferric alum". Techn. Bull. New South Wales Dept of Agriculture. 19: 1–16.
- ↑ Spolaore P, Joannis-Cassan C, Duran E, Isambert A; Joannis-Cassan; Duran; Isambert (2006). "Commercial applications of microalgae". J. Biosci. Bioeng. 101 (2): 87–96. doi:10.1263/jbb.101.87. PMID 16569602.
- ↑ Christaki, E.; Florou-Paneri, P.; Bonos, E. (2011). "Microalgae: A novel ingredient in nutrition". International Journal of Food Sciences and Nutrition. 62 (8): 794–799. doi:10.3109/09637486.2011.582460. PMID 21574818.
- ↑ Ku, C. S.; Pham, T. X.; Park, Y.; Kim, B.; Shin, M.; Kang, I.; Lee, J. (2013). "Edible blue-green algae reduce the production of pro-inflammatory cytokines by inhibiting NF-κB pathway in macrophages and splenocytes". Biochimica et Biophysica Acta. 1830 (4): 2981–8. doi:10.1016/j.bbagen.2013.01.018. PMID 23357040.
- ↑ Mišurcová, L.; Škrovánková, S. A.; Samek, D. A.; Ambrožová, J.; Machů, L. (2012). "Health Benefits of Algal Polysaccharides in Human Nutrition". Advances in Food and Nutrition Research Volume 66. Advances in Food and Nutrition Research. 66. pp. 75–145. doi:10.1016/B978-0-12-394597-6.00003-3. ISBN 9780123945976. PMID 22909979.
Further reading
- Gillian Cribbs (1997), Nature's Superfood: the Blue-Green Algae Revolution, Newleaf, ISBN 0-7522-0569-2.
- Marshall Savage (1992, 1994), The Millennial Project: Colonizing the Galaxy in Eight Easy Steps, Little, Brown, ISBN 0-316-77163-5.
- Fogg, G.E., Stewart, W.D.P., Fay, P. and Walsby, A.E. (1973), The Blue-green Algae, Academic Press, London and New York, ISBN 0-12-261650-2.
- "Architects of the earth's atmosphere", Introduction to the Cyanobacteria, University of California, Berkeley, 3 February 2006.
- Whitton, B. A., Phylum Cyanophyta (Cyanobacteria), in The Freshwater Algal Flora of the British Isles, Cambridge, Cambridge University Press, ISBN 0-521-77051-3.
- Pentecost A., Franke U.; Franke (2010). "Photosynthesis and calcification of the stromatolitic freshwater cyanobacterium Rivularia". Eur. J. Phycol. 45 (4): 345–353. doi:10.1080/09670262.2010.492914..
- Whitton, B. A. and Potts, M. (Eds) (2000), The Ecology of Cyanobacteria: their Diversity in Time and Space, Springer, ISBN 0-7923-4735-8.
- Whitton, B. A. (Ed) (2012) Ecology of Cyanobacteria II: Their Diversity in Space and Time Springer Science & Business Media. ISBN 9789400738553.
- "From Micro-Algae to Blue Oil", ParisTech Review, December 2011.
External links
| Wikimedia Commons has media related to Cyanobacteria. |
- What are Cyanobacteria and What are its Types?
- Webserver for Cyanobacteria Research
- CyanoBase
- Growth Model for the Blue-Green Alga Anabaena catenula Wolfram Demonstrations Project—requires CDF player (free)
- Diving an Antarctic Time Capsule Filled With Primordial Life
- BgaGenomicsDB
![]() This article incorporates text available under the CC BY 2.5 license.
This article incorporates text available under the CC BY 2.5 license.