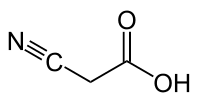
Cyanoacetic acid
 | |
| Names | |
|---|---|
| IUPAC name
2-cyanoacetic acid | |
| Identifiers | |
| 372-09-8 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 9357 |
| ECHA InfoCard | 100.006.131 |
| PubChem | 9740 |
| |
| |
| Properties | |
| C3H3NO2 | |
| Molar mass | 85.06 g/mol |
| Appearance | colorless solid |
| Density | 1.287 g/cm3 |
| Melting point | 69-70 ℃ |
| Boiling point | 108 ℃ (15 mm Hg) |
| 1000 g/L (20 ℃) in water | |
| Hazards | |
| Main hazards | C:Corrosive; HazardClass:8 |
| Flash point | 107 °C (225 °F; 380 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cyanoacetic acid is an organic compound. It is a white, hygroscopic solid. The compound contains two functional groups, a nitrile and a carboxylic acid. It is a precursor to cyanoacrylates, components of adhesives.[1]
Preparation and reactions
Cyanoacetic acid is prepared by treatment of chloroacetate salts with sodium cyanide followed by acidification.[1][2] Electrosynthesis by cathodic reduction of carbon dioxide and anodic oxidation of acetonitrile also affords cyanoacetic acid.[3]
Cyanoacetic acid is used to do cyanoacetylation, first convenient method described by J. Slätt.[4]
It is about 1000x more acidic than acetic acid, with a pKa of 2.5. Upon heating at 160 °C, it undergoes decarboxylation to give acetonitrile:
- C3H3NO2 → C2H3N + CO2
Applications
The largest scale reaction is its esterification to give ethyl cyanoacrylate. As of 2007, more than 10,000 tons of cyanoacetic acid were produced annually.
Cyanoacetic acid is a versatile intermediate in the preparation of chemicals. it is a precursor to synthetic caffeine via the intermediacy of theophylline. It is a building block for many drugs, including dextromethorphan, amiloride, sulfadimethoxine, and allopurinol,[1] and also for Peldesine.
Safety
The LD50 (oral, rats) is 1.5 g/kg.[1]
References
- 1 2 3 4 Harald Strittmatter, Stefan Hildbrand and Peter Pollak Malonic Acid and Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry 2007, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a16_063.pub2
- ↑ Inglis, J. K. H. (1928). "Ethyl Cyanoacetate". Organic Syntheses. 8: 74. doi:10.15227/orgsyn.008.0074.
- ↑ Barba, Fructuoso; Batanero, Belen (2004). "Paired Electrosynthesis of Cyanoacetic Acid". The Journal of Organic Chemistry. 69 (7): 2423-2426. doi:10.1021/jo0358473.
- ↑ Bergman, Jan; Romero, Ivan; Slätt, Johnny (2004). "Cyanoacetylation of indoles, pyrroles and aromatic amines with the combination cyanoacetic acid and acetic anhydride". Synthesis. 16: 2760-2765. doi:10.1055/s-2004-831164.