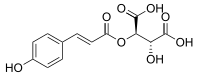
Coutaric acid
 | |
| Names | |
|---|---|
| IUPAC name
(2R,3R)-2-Hydroxy-3-(((E)-3-(4-hydroxyphenyl)acryloyl)oxy)succinic acid | |
| Other names
trans-coutaric acid cis-coutaric acid trans-p-Coumaroyltartaric acid cis-p-coumaroyl-(+)-tartaric acid trans-p-coumaroyl-(+)-tartaric acid cis-Coumaroyl tartaric acidbr>trans-Coumaroyl tartaric acid | |
| Identifiers | |
| 27174-07-8 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:77439 |
| ChemSpider | 26325199 |
| |
| |
| Properties | |
| C13H12O8 | |
| Molar mass | 296.23 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Coutaric acid is an hydroxycinnamoyltartaric acid found in wine, pomace[1] and grape.[2] It is an ester formed from coumaric acid and tartaric acid.
References
- ↑ Maier, T.; Sanzenbacher, S.; Kammerer, D. R.; Berardini, N.; Conrad, J. R.; Beifuss, U.; Carle, R.; Schieber, A. (2006). "Isolation of hydroxycinnamoyltartaric acids from grape pomace by high-speed counter-current chromatography". Journal of Chromatography A. 1128 (1–2): 61–67. doi:10.1016/j.chroma.2006.06.082. PMID 16860334.
- ↑ Singleton, V. L.; Zaya, J.; Trousdale, E. K. (1986). "Caftaric and coutaric acids in fruit of Vitis". Phytochemistry. 25 (9): 2127. doi:10.1016/0031-9422(86)80078-4.
This article is issued from Wikipedia - version of the 9/4/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.