Coomber's relationship
Coomber's relationship can be used to describe how the internal pressure and dielectric constant of a non-polar liquid are related.
As  , which defines the internal pressure of a liquid, it can be found that:
, which defines the internal pressure of a liquid, it can be found that:
where  is equal to the number of molecules
is equal to the number of molecules
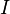 is the ionization potential of the liquid
is the ionization potential of the liquid
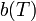 is a temperature dependent relation based on numerical constants of the pair summation from inter-particle geometry
is a temperature dependent relation based on numerical constants of the pair summation from inter-particle geometry
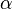 is the polarizability
is the polarizability
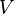 is the volume of the liquid
is the volume of the liquid
where for most non-polar liquids 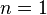
References
- Meeten, G.H., "Theoretical Basis for Coomber's Relationship", Nature Vol. 223, August 23, 1969
This article is issued from Wikipedia - version of the 3/17/2013. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
