Nerve conduction velocity
Nerve conduction velocity is an important aspect of nerve conduction studies. It is the speed at which an electrochemical impulse propagates down a neural pathway. Conduction velocities are affected by a wide array of factors, including age, sex, and various medical conditions. Studies allow for better diagnoses of various neuropathies, especially demyelinating conditions as these conditions result in reduced or non-existent conduction velocities.
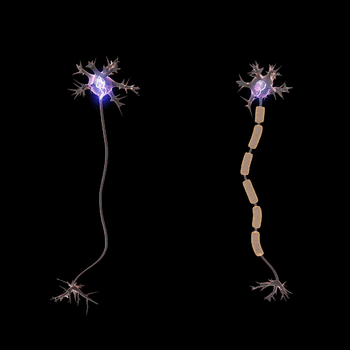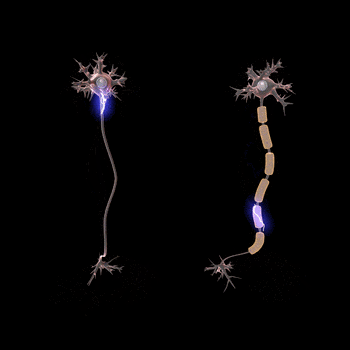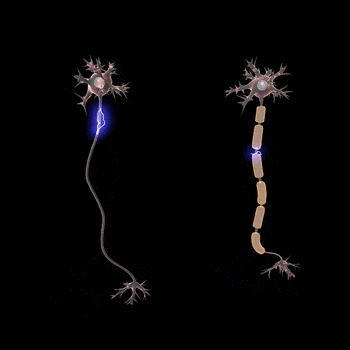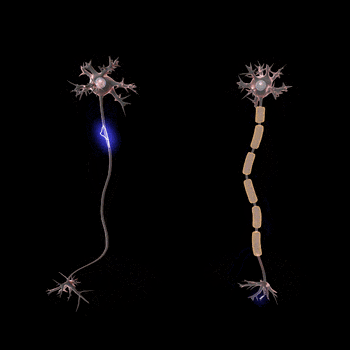
Normal conduction velocities
Ultimately, conduction velocities are specific to each individual and depend largely on an axon's diameter and the degree to which that axon is myelinated, but the majority of 'normal' individuals fall within defined ranges.[1]
Nerve impulses are extremely slow compared to the speed of electrical impulses which are on the order of 50%–99% of the speed of light, however, very fast compared to the speed of blood flow, with some myelinated neurons conducting at speeds up to 120 m/s (432 km/h or 275 mph).
| Type | Erlanger-Gasser Classification | Diameter | Myelin | Conduction velocity | Associated muscle fibers |
|---|---|---|---|---|---|
| α | Aα | 13-20 µm | Yes | 80–120 m/s | Extrafusal muscle fibers |
| γ | Aγ | 5-8 µm | Yes | 4–24 m/s [2][3] | Intrafusal muscle fibers |
Different sensory receptors are innervated by different types of nerve fibers. Proprioceptors are innervated by type Ia, Ib and II sensory fibers, mechanoreceptors by type II and III sensory fibers, and nociceptors and thermoreceptors by type III and IV sensory fibers.
| Type | Erlanger-Gasser Classification | Diameter | Myelin | Conduction velocity | Associated sensory receptors |
|---|---|---|---|---|---|
| Ia | Aα | 13-20 µm | Yes | 80–120 m/s[4] | Responsible for proprioception |
| Ib | Aα | 13-20 µm | Yes | 80–120 m/s | Golgi tendon organ |
| II | Aβ | 6-12 µm | Yes | 33–75 m/s | Secondary receptors of muscle spindle All cutaneous mechanoreceptors |
| III | Aδ | 1-5 µm | Thin | 3–30 m/s | Free nerve endings of touch and pressure Nociceptors of neospinothalamic tract Cold thermoreceptors |
| IV | C | 0.2-1.5 µm | No | 0.5-2.0 m/s | Nociceptors of paleospinothalamic tract Warmth receptors |
| Type | Erlanger-Gasser Classification | Diameter | Myelin | Conduction velocity |
|---|---|---|---|---|
| preganglionic fibers | B | 1-5 µm | Yes | 3–15 m/s |
| postganglionic fibers | C | 0.2-1.5 µm | No | 0.5-2.0 m/s |
| Nerve | Conduction velocity[5][6] |
|---|---|
| Median Sensory | 45–70 m/s |
| Median Motor | 49–64 m/s |
| Ulnar Sensory | 48–74 m/s |
| Ulnar Motor | 49+ m/s |
| Peroneal Motor | 44+ m/s |
| Tibial Motor | 41+ m/s |
| Sural Sensory | 46–64 m/s |
Normal impulses in peripheral nerves of the legs travel at 40–45 m/s, and 50–65 m/s in peripheral nerves of the arms.[7] Largely generalized, normal conduction velocities for any given nerve will be in the range of 50–60 m/s.[8]
Testing methods

Nerve conduction studies
Nerve Conduction Velocity is just one of many measurements commonly made during a nerve conduction study (NCS). The purpose of these studies is to determine whether nerve damage is present and how severe that damage may be.
Nerve conduction studies are performed as follows:[8]
- Two electrodes are attached to the subject's skin over the nerve being tested.
- Electrical impulses are sent through one electrode to stimulate the nerve.
- The second electrode records the impulse sent through the nerve as a result of stimulation.
- The time difference between stimulation from the first electrode and pick-up by the downstream electrode is known as the latency. Nerve conduction latencies are typically on the order of milliseconds.
Although conduction velocity itself is not directly measured, calculating conduction velocities from NCS measurements is trivial. The distance between the stimulating and receiving electrodes is divided by the impulse latency, resulting in conduction velocity.
Many times, Needle EMG is also performed on subjects at the same time as other NCS procedures because they aid in detecting whether muscles are functioning properly in response to stimuli sent via their connecting nerves.[8] EMG is the most important component of electrodiagnosis of motor neuron diseases as it often leads to the identification of motor neuron involvement before clinical evidence can be seen.[9]
Micromachined 3D electrode arrays
Typically, the electrodes used in an EMG are stuck to the skin over a thin layer of gel/paste.[8] This allows for better conduction between electrode and skin. However, as these electrodes do not pierce the skin, there are impedances that result in erroneous readings, high noise levels, and low spatial resolution in readings.[10]
To address these problems, new devices are being developed, such as 3-dimensional electrode arrays. These are MEMS devices that consist of arrays of metal micro-towers capable of penetrating the outer layers of skin, thus reducing impedance.[10]
Compared with traditional wet electrodes, multielectrode arrays offer the following:[10]
- Electrodes are about 1/10 the size of standard wet surface electrodes
- Arrays of electrodes can be created and scaled to cover areas of almost any size
- Reduced impedance
- Improved signal power
- Higher amplitude signals
- Allow better real-time nerve impulse tracking
Causes of conduction velocity deviations
Anthropometric and other individualized factors
Baseline nerve conduction measurements are different for everyone, as they are dependent upon the individual's age, sex, local temperatures, and other anthropometric factors such as hand size and height.[5][11] It is important to understand the effect of these various factors on the normal values for nerve conduction measurements to aid in identifying abnormal nerve conduction study results. The ability to predict normal values in the context of an individual's anthropometric characteristics increases the sensitivities and specificities of electrodiagnostic procedures.[5]
Age
Normal 'adult' values for conduction velocities are typically reached by age 4. Conduction velocities in newborns and toddlers tend to be about half the adult values.[1]
Nerve conduction studies performed on healthy adults revealed that age is negatively associated with the sensory amplitude measures of the Median, Ulnar, and Sural nerves. Negative associations were also found between age and the conduction velocities and latencies in the Median sensory, Median motor, and Ulnar sensory nerves. However, conduction velocity of the Sural nerve is not associated with age. In general, conduction velocities in the upper extremities decrease by about 1 m/s for every 10 years of age.[5]
Sex
Sural nerve conduction amplitude is significantly smaller in females than males, and the latency of impulses is longer in females, thus a slower conduction velocity.[5]
Other nerves have not been shown to exhibit any gender biases.
Temperature
In general, the conduction velocities of most motor and sensory nerves are positively and linearly associated with body temperature (low temperatures slow nerve conduction velocity and higher temperatures increase conduction velocity).[1]
Conduction velocities in the Sural nerve seem to exhibit an especially strong correlation with the local temperature of the nerve.[5]
Height
Conduction velocities in both the Median sensory and Ulnar sensory nerves are negatively related to an individual's height, which likely accounts for the fact that, among most of the adult population, conduction velocities between the wrist and digits of an individual's hand decrease by 0.5 m/s for each inch increase in height.[5] As a direct consequence, impulse latencies within the Median, Ulnar, and Sural nerves increases with height.[5]
The correlation between height and the amplitude of impulses in the sensory nerves is negative.[5]
Hand factors
Circumference of the index finger appears to be negatively associated with conduction amplitudes in the Median and Ulnar nerves. In addition, people with larger wrist ratios (anterior-posterior diameter : medial-lateral diameter) have lower Median nerve latencies and faster conduction velocities.[5]
Medical conditions
Amyotrophic lateral sclerosis (ALS)
Amyotrophic Lateral Sclerosis (ALS) aka 'Lou Gehrig's disease' is a progressive and inevitably fatal neurodegenerative disease affecting the motor neurons.[9] Because ALS shares many symptoms with other neurodegenerative diseases, it can be difficult to diagnose properly. The best method of establishing a confident diagnosis is via electrodiagnostic evaluation. To be specific, motor nerve conduction studies of the Median, Ulnar, and peroneal muscles should be performed, as well as sensory nerve conduction studies of the Ulnar and Sural nerves.[9]
In patients with ALS, it has been shown that distal motor latencies and slowing of conduction velocity worsened as the severity of their muscle weakness increased. Both symptoms are consistent with the axonal degeneration occurring in ALS patients.[9]
Carpal tunnel syndrome
Carpal Tunnel Syndrome (CTS) is a form of nerve compression syndrome caused by the compression of the median nerve at the wrist. Typical symptoms include numbness, tingling, burning pains, or weakness in the hand.[12][13] CTS is another condition for which electrodiagnostic testing is valuable.[12][14] However, before subjecting a patient to nerve conduction studies, both Tinel's test and Phalen's test should be performed. If both results are negative, it is very unlikely that the patient has CTS, and further testing is unnecessary.[13]
Carpal tunnel syndrome presents in each individual to different extents. Measurements of nerve conduction velocity are critical to determining the degree of severity.[14][15] These levels of severity are categorized as:[12][13]
- Mild CTS: Prolonged sensory latencies, very slight decrease in conduction velocity. No suspected axonal degeneration.
- Moderate CTS: Abnormal sensory conduction velocities and reduced motor conduction velocities. No suspected axonal degeneration.
- Severe CTS: Absence of sensory responses and prolonged motor latencies (reduced motor conduction velocities).
- Extreme CTS: Absence of both sensory and motor responses.
One common electrodiagnostic measurement includes the difference between sensory nerve conduction velocities in the pinkie finger and index finger. In most instances of CTS, symptoms will not present until this difference is greater than 8 m/s.[12][13]
Guillain–Barré syndrome
Guillain–Barré syndrome (GBS) is a peripheral neuropathy involving the degeneration of myelin sheathing and/or nerves that innervate the head, body, and limbs.[7] This degeneration is due to an autoimmune response typically initiated by various infections.
Two primary classifications exist: demyelinating (Schwann cell damage) and axonal (direct nerve fiber damage).[7][16] Each of these then branches into additional sub-classifications depending on the exact manifestation. In all cases, however, the condition results in weakness or paralysis of limbs, the potentially fatal paralysis of respiratory muscles, or a combination of these effects.[7]
The disease can progress very rapidly once symptoms present (severe damage can occur within as little as a day).[7] Because electrodiagnosis is one of the fastest and most direct methods of determining the presence of the illness and its proper classification, nerve conduction studies are extremely important.[16] Without proper electrodiagnostic assessment, GBS is commonly misdiagnosed as Polio, West Nile virus, Tick paralysis, various Toxic neuropathies, CIDP, Transverse myelitis, or Hysterical paralysis.[7] Two sets of nerve conduction studies should allow for proper diagnosis of Guillain–Barré syndrome. It is recommended that these be performed within the first 2 weeks of symptom presentation and again sometime between 3 and 8 weeks.[16]
Electrodiagnostic findings that may implicate GBS include:[6][7][16]
- Complete conduction blocks
- Abnormal or absent F waves
- Attenuated compound muscle action potential amplitudes
- Prolonged motor neuron latencies
- Severely slowed conduction velocities (sometimes below 20 m/s)
Lambert-Eaton myasthenic syndrome
Lambert–Eaton myasthenic syndrome (LEMS) is an autoimmune disease in which auto-antibodies are directed against voltage-gated calcium channels at presynaptic nerve terminals. Here, the antibodies inhibit the release of neurotransmitters, resulting in muscle weakness and autonomic dysfunctions.[17]
Nerve conduction studies performed on the Ulnar motor and sensory, Median motor and sensory, Tibial motor, and Peroneal motor nerves in patients with LEMS have shown that the conduction velocity across these nerves is actually normal. However, the amplitudes of the compound motor action potentials may be reduced by up to 55%, and the duration of these action potentials decreased by up to 47%.[17]
Peripheral diabetic neuropathy
At least half the population with Diabetes mellitus is also affected with Diabetic neuropathy, causing numbness and weakness in the peripheral limbs.[18] Studies have shown that the Rho/Rho-kinase signaling pathway is more active in individuals with diabetes and that this signaling activity occurs mainly in the Nodes of Ranvier and Schmidt-Lanterman incisures.[18] Therefore, over-activity of the Rho/Rho-kinase signaling pathway may inhibit nerve conduction.
Motor nerve conduction velocity studies revealed that conductance in diabetic rats was about 30% lower than that of the non-diabetic control group. In addition, activity along the Schmidt-Lanterman incisures was non-continuous and non-linear in the diabetic group, but linear and continuous in the control. These deficiencies were eliminated after the administration of Fasudil to the diabetic group, implying that it may be a potential treatment.[18]
See also
References
- 1 2 3 "Nerve conduction velocity". National Institutes of Health. 31 October 2013. Retrieved 13 November 2013.
- ↑ Andrew BL, Part NJ (1972) Properties of fast and slow motor units in hind limb and tail muscles of the rat. Q J Exp Physiol Cogn Med Sci 57:213-225.
- ↑ Russell NJ (1980). "Axonal conduction velocity changes following muscle tenotomy or deafferentation during development in the rat". J Physiol. 298: 347–360.
- ↑ Siegel, Allan; Sapru, Hreday (2005). Essential Neuroscience. p. 257. ISBN 978-0781750776.
- 1 2 3 4 5 6 7 8 9 10 Stetson, PhD, Diana S.; James W. Albers; Barbara A. Silverstein; Robert A. Wolfe (October 1992). "Effects of Age, Sex, and Anthropometric Factors on Nerve Conduction Measures". Muscle & Nerve. 15: 1095–1104. doi:10.1002/mus.880151007.
- 1 2 Sedano, Maria J.; Canga, Ana, Pablos, Carmen, Polo, Jose M., Berciano, Jose (31 January 2013). "Muscle MRI in severe Guillain–Barré syndrome with motor nerve inexcitability". Journal of Neurology. 260 (6): 1624–1630. doi:10.1007/s00415-013-6845-y. Cite uses deprecated parameter
|coauthors=(help) - 1 2 3 4 5 6 7 Parry, Gareth J. (2007). Guillain–Barré Syndrome. New York, NY: Demos Medical Publishing. pp. 1–9. ISBN 978-1-932603-56-9.
- 1 2 3 4 "Nerve Conduction Study (NCS)". Johns Hopkins Medicine. Retrieved 17 November 2013.
- 1 2 3 4 Joyce, Nanette C.; Carter, Gregory T. (May 2013). "Electrodiagnosis in Persons With Amyotrophic Lateral Sclerosis". PM&R. 5 (5, Supplement): S89–S95. doi:10.1016/j.pmrj.2013.03.020.
- 1 2 3 Rajaraman, Swaminathan; Bragg, Julian A.; Ross, James D.; Allen, Mark G. (30 June 2011). "Micromachined three-dimensional electrode arrays for transcutaneous nerve tracking". Journal of Micromechanics and Microengineering. 21 (8). doi:10.1088/0960-1317/21/8/085014.
- ↑ Thanakiatpinyo, MD, Thanitta; Gulapar Srisawasdi (2013). "Effect of Hand Size on the Stimulation Intensities Required for Median and Ulnar Sensory Nerve Conduction Studies". Archives of Physical Medicine and Rehabilitation. 94: 925–929. doi:10.1016/j.apmr.2012.11.029.
- 1 2 3 4 Werner, Robert A.; Andary, Michael (October 2011). "Electrodiagnostic evaluation of carpal tunnel syndrome". Muscle & Nerve. 44 (4): 597–607. doi:10.1002/mus.22208.
- 1 2 3 4 Ntani, Georgia; Palmer, Keith T., Linaker, Cathy, Harris, E Clare, Van der Star, Richard, Cooper, Cyrus, Coggon, David (15 August 2013). "Symptoms, signs and nerve conduction velocities in patients with suspected carpal tunnel syndrome". BMC Musculoskeletal Disorders. 14 (1): 1–10. doi:10.1186/1471-2474-14-242. Cite uses deprecated parameter
|coauthors=(help) - 1 2 Inukai, Tomoo; Uchida, Kenzo; Kubota, Chikara; Takamura, Takaharu; Nakajima, Hideaki; Baba, Hisatoshi (24 October 2013). "Second lumbrical-interossei nerve test predicts clinical severity and surgical outcome of carpal tunnel syndrome". Journal of Clinical Neuroscience. 20 (9): 1224–1227. doi:10.1016/j.pmrj.2013.04.007.
- ↑ Robinson, Lawrence, R.; Strakowski, Jeffrey; Kennedy, David J. (May 2013). "Is the Combined Sensory (Robinson) Index Routinely Indicated for All Cases of Suspected Carpal Tunnel Syndrome Undergoing Electrodiagnostic Evaluation?". PM&R. 5 (5): 433–437. doi:10.1016/j.pmrj.2013.04.007.
- 1 2 3 4 Shahrizaila, Nortina; Goh, Khean Jin; Abdullah, Suhailah; Kuppusamy, Rishikesan; Yuki, Nobuhiro (8 February 2013). "Two sets of nerve conduction studies may suffice in reaching a reliable electrodiagnosis in Guillain–Barré syndrome". Clinical Neurophysiology. 124 (7): 1456–1459. doi:10.1016/j.clinph.2012.12.047.
- 1 2 Crone, Clarissa; Christiansen, Ingelise; Vissing, John (3 May 2013). "Myopathic EMG findings and type II muscle fiber atrophy in patients with Lambert-Eaton myasthenic syndrome". Clinical Neurophysiology. 124 (9): 1889–1892. doi:10.1016/j.clinph.2013.02.115.
- 1 2 3 Kanazawa, Yasushi; Junko Takahashi-Fujigasaki; Sho Ishizawa; Naoko Takabayashi; Kumiko Ishibashi; Keiichiro Matoba; Daiji Kawanami; Tamotsu Yokota; Naoko Tajima; Kazunori Utsunomiya (September 2013). "The Rho-kinase inhibitor fasudil restores normal motor nerve conduction velocity in diabetic rats by assuring the proper localization of adhesion-related molecules in myelinating Schwann cells". Experimental Neurology. 247: 438–446. doi:10.1016/j.expneurol.2013.01.012.