Cln3
| G1/S-specific cyclin CLN3 | |
|---|---|
| Identifiers | |
| Organism | |
| Symbol | CLN3 |
| Alt. symbols | YAL040C, WHI1, DAF1, FUN10 |
| Entrez | 851191 |
| RefSeq (mRNA) | NM_001178185 |
| RefSeq (Prot) | NP_009360 |
| UniProt | P13365 |
| Other data | |
| Chromosome | 1: 0.07 - 0.07 Mb |
G1/S-specific cyclin Cln3 is a protein that is encoded by the CLN3 gene. The Cln3 protein is a budding yeast G1 cyclin that controls the timing of Start, the point of commitment to a mitotic cell cycle. It is an upstream regulator of the other G1 cyclins,[1] and it is thought to be the key regulator linking cell growth to cell cycle progression.[2][3] It is a 65 kD, unstable protein;[4] like other cyclins, it functions by binding and activating cyclin-dependent kinase (CDK).[5]
Cln3 in Start regulation
Cln3 regulates Start, the point at which budding yeast commit to the G1/S transition and thus a round of mitotic division. It was first identified as a gene controlling this process in the 1980s; research over the past few decades has provided a mechanistic understanding of its function.
Identification of CLN3 gene
The CLN3 gene was originally identified as the whi1-1 allele in a screen for small size mutants of Saccharomyces cerevisiae (for Cln3's role in size control, see below).[6][7] This screen was inspired by a similar study in Schizosaccharomyces pombe, in which the Wee1 gene was identified as an inhibitor of cell cycle progression that maintained normal cell size.[8] Thus, the WHI1 gene was at first thought to perform a size control function analogous to that of Wee1 in pombe. However, it was later found that WHI1 was in fact a positive regulator of Start, as its deletion caused cells to delay in G1 and grow larger than wild-type cells.[9][10] The original WHI1-1 allele (changed from whi1-1 because it is a dominant allele) in fact contained a nonsense mutation that removed a degradation-promoting PEST sequence from the Whi1 protein and thus accelerated G1 progression.[4][9] WHI1 was furthermore found to be a cyclin homologue,[9] and it was shown that simultaneous deletion of WHI1—renamed CLN3—and the previously identified G1 cyclins, CLN1 and CLN2, caused permanent G1 arrest.[11][12] This showed that the three G1 cyclins were responsible for controlling Start entry in budding yeast.
G1-S transition
The three G1 cyclins collaborate to drive yeast cells through the G1-S transition, i.e. to enter S-phase and begin DNA replication. The current model of the gene regulatory network controlling the G1-S transition is shown in Figure 1.
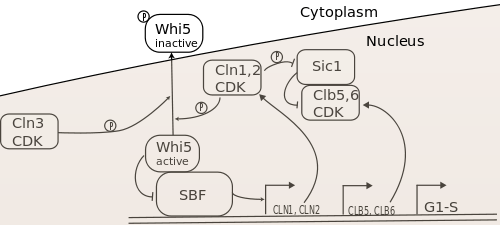
The key targets of the G1 cyclins in this transition are the transcription factors SBF and MBF (not shown in the diagram),[13][14][15][16] as well as the B-type cyclin inhibitor Sic1.[17] Cln-CDKs activate SBF by phosphorylating and promoting nuclear export of its inhibitor, Whi5, which associates with promoter-bound SBF.[18][19][20][21][22] The precise mechanism of MBF activation is unknown. Together, these transcription factors promote the expression of over 200 genes, which encode the proteins necessary for carrying out the biochemical activities of S-phase.[23][24] These include the S-phase cyclins Clb5 and Clb6, which bind CDK to phosphorylate S-phase targets. However, Clb5,6-CDK complexes are inhibited by Sic1, so S-phase initiation requires phosphorylation and degradation of Sic1 by Cln1,2-CDK to proceed fully.[17]
Cln3 activates a Cln1,2 positive feedback loop
Although all three G1 cyclins are necessary for normal regulation of Start and the G1-S transition, Cln3 activity seems to be the deciding factor in S-phase initiation, with Cln1 and Cln2 serving to actuate the Cln3-based decision to transit Start. It was found early on that Cln3 activity induced expression of Cln1 and Cln2. Furthermore, Cln3 was a stronger activator Start transit than Cln1 and Cln2, even though Cln3-CDK had an inherently weaker kinase activity than the other Clns. This indicated that Cln3 was an upstream regulator of Cln1 and Cln2.[1] Furthermore, it was found, as shown in Figure 1, that Cln1 and Cln2 could activate their own transcription via SBF, completing a positive feedback loop that could contribute to rapid activation and S-phase entry.[25][26] Thus, Start transit seems to rely on reaching a sufficient level of Cln3-CDK activity to induce the Cln1,2 positive feedback loop, which rapidly increases SBF/MBF and Cln1,2 activity, allowing a switch-like G1-S transition. The role of positive feedback in this process has been challenged,[27][28] but recent experiments have confirmed its importance for rapid inactivation and nuclear export of Whi5,[29] which is the molecular basis of commitment to S-phase.[30]
Cln3 and cell size control
As discussed above, Cln3 was originally identified as a regulator of budding yeast cell size. The elucidation of the mechanisms by which it regulates Start has revealed a means for it to link cell size to cell cycle progression, but questions remain as to how it actually senses cell size.
Start requires a threshold cell size
The simple observation that cells of a given type are similar in size, and the question of how this similarity is maintained, has long fascinated cell biologists. The study of cell size control in budding yeast began in earnest in the mid 1970s, when the regulation of the budding yeast cell cycle was first being elucidated by Lee Hartwell and colleagues. Seminal work in 1977 found that yeast cells maintain a constant size by delaying their entry into the cell cycle (as assayed by budding) until they have grown to a threshold size.[31][32] Later worked refined this result to show that Start specifically, rather than some other aspect of the G1-S transition, is controlled by the size threshold.[33]
Translational size sensing
That Start transit requires the attainment of a threshold cell size directly implies that yeast cells measure their own size, so that they can use that information to regulate Start. A favored model for how yeast cells, as well as cells of other species, measure their size relies on the detection of overall translation rate. Essentially, since cell growth consists, to a great extent, of the synthesis of ribosomes to produce more proteins, the overall rate of protein production should reflect cell size. Thus, a single protein that is produced at a constant rate relative to total protein production capacity will be produced in higher quantities as the cell grows. If this protein promotes cell cycle progression (Start in the case of yeast), then it will link cell cycle progression to translation rate and, therefore, cell size. Importantly, this protein must be unstable, so that its levels depend on its current translation rate, rather than the rate of translation over time.[34] Furthermore, since the cell grows in volume as well as mass, the concentration of this size sensor will remain constant with growth, so its activity must be compared against something that does not change with cell growth. Genomic DNA was suggested as such a standard early on,[35] because it is (by definition) present in a constant quantity until the start of DNA replication. How this occurs remains a major question in current studies of size control (see below).
Before the identification of Cln3 and its function, accrued evidence indicated that such translational size sensing operated in yeast. First, it was confirmed that the total rate of protein synthesis per cell increases with growth,[36] a fundamental prerequisite for this model. It was later shown that treatment with the protein synthesis inhibitor cycloheximide delayed Start in yeast, indicating that translation rate controlled Start.[37][38] Finally, it was also shown that this delay occurred even with short pulses of cycloheximide, confirming that an unstable activating protein was required for Start.[39]
Cln3 as size sensor
The model of budding yeast size control, in which a threshold size for Start entry is detected by a translational size sensor, required a "sizer" protein; the properties of Cln3 made it the prime candidate for that role from the time of its discovery. First, it was a critical Start activator, as G1 length varied inversely with Cln3 expression and activity levels.[9] Second, it was expressed nearly constitutively throughout the cell cycle and in G1 in particular[1]—unusual for cyclins, which (as their name suggests) oscillate in expression with the cell cycle. These two properties meant that Cln3 could serve as a Start activator that depended on total translation rate. Finally, Cln3 was also shown to be highly unstable, the third necessary property of a translational sizer (as discussed above).[4][5]
Thus, Cln3 seems to be the size sensor in budding yeast, as it exhibits the necessary properties of a translational sizer and is the most upstream regulator of Start. A critical question remains, however, as to how its activity is rendered size dependent. As noted above, any translational size sensor should be at constant concentration, and thus constant activity, in the cytoplasm as cells grow. In order to detect its size, the cell must compare the absolute number of sizer molecules to some non-growing standard, with the genome the obvious choice for such a standard. It was originally thought that yeast accomplished this with Cln3 by localizing it (and its target, Whi5) to the nucleus: nuclear volume was assumed to scale with genome content, so that an increasing concentration of Cln3 in the nucleus could indicate increasing Cln3 molecules relative to the genome.[2][40][41] However, the nucleus has recently been shown to grow during G1, irrespective of genome content, undermining this model.[42] Recent experiments have suggested that Cln3 activity could be titrated directly against genomic DNA, through its DNA-bound interaction with SBF-Whi5 complexes.[43] Finally, other models exist that do not rely on comparison of Cln3 levels to DNA. One posits a non-linear relationship between total translation rate and Cln3 translation rate caused by an Upstream open reading frame;[44] another suggests that the increase in Cln3 activity at the end of G1 relies on competition for the chaperone protein Ydj1, which otherwise holds Cln3 molecules in the Endoplasmic reticulum.[45]
References
- 1 2 3 Tyers, M; Tokiwa, G; Futcher, B (May 1993). "Comparison of the Saccharomyces cerevisiae G1 cyclins: Cln3 may be an upstream activator of Cln1, Cln2 and other cyclins.". The EMBO Journal. 12 (5): 1955–68. PMC 413417
 . PMID 8387915.
. PMID 8387915. - 1 2 Futcher, B (Dec 1996). "Cyclins and the wiring of the yeast cell cycle.". Yeast. 12 (16): 1635–46. doi:10.1002/(SICI)1097-0061(199612)12:16<1635::AID-YEA83>3.0.CO;2-O. PMID 9123966.
- ↑ Jorgensen, P; Tyers, M (2004-12-14). "How cells coordinate growth and division.". Current Biology. 14 (23): R1014–27. doi:10.1016/j.cub.2004.11.027. PMID 15589139.
- 1 2 3 Tyers, M; Tokiwa, G; Nash, R; Futcher, B (May 1992). "The Cln3-Cdc28 kinase complex of S. cerevisiae is regulated by proteolysis and phosphorylation.". The EMBO Journal. 11 (5): 1773–84. PMC 556635
 . PMID 1316273.
. PMID 1316273. - 1 2 Cross, FR; Blake, CM (Jun 1993). "The yeast Cln3 protein is an unstable activator of Cdc28.". Molecular and Cellular Biology. 13 (6): 3266–71. PMC 359776
 . PMID 8497251.
. PMID 8497251. - ↑ Sudbery, PE; Goodey, AR; Carter, BL (1980-11-27). "Genes which control cell proliferation in the yeast Saccharomyces cerevisiae.". Nature. 288 (5789): 401–4. doi:10.1038/288401a0. PMID 7001255.
- ↑ Carter, BL; Sudbery, PE (Nov 1980). "Small-sized mutants of Saccharomyces cerevisiae.". Genetics. 96 (3): 561–6. PMC 1214361
 . PMID 7021310.
. PMID 7021310. - ↑ Nurse, P (1975-08-14). "Genetic control of cell size at cell division in yeast.". Nature. 256 (5518): 547–51. doi:10.1038/256547a0. PMID 1165770.
- 1 2 3 4 Nash, R; Tokiwa, G; Anand, S; Erickson, K; Futcher, AB (1988-12-20). "The WHI1+ gene of Saccharomyces cerevisiae tethers cell division to cell size and is a cyclin homolog.". The EMBO Journal. 7 (13): 4335–46. PMC 455150
 . PMID 2907481.
. PMID 2907481. - ↑ Cross, FR (Nov 1988). "DAF1, a mutant gene affecting size control, pheromone arrest, and cell cycle kinetics of Saccharomyces cerevisiae.". Molecular and Cellular Biology. 8 (11): 4675–84. PMC 365557
 . PMID 3062366.
. PMID 3062366. - ↑ Richardson, HE; Wittenberg, C; Cross, F; Reed, SI (1989-12-22). "An essential G1 function for cyclin-like proteins in yeast.". Cell. 59 (6): 1127–33. doi:10.1016/0092-8674(89)90768-X. PMID 2574633.
- ↑ Hadwiger, JA; Wittenberg, C; Richardson, HE; de Barros Lopes, M; Reed, SI (Aug 1989). "A family of cyclin homologs that control the G1 phase in yeast.". Proceedings of the National Academy of Sciences of the United States of America. 86 (16): 6255–9. doi:10.1073/pnas.86.16.6255. PMC 297816
 . PMID 2569741.
. PMID 2569741. - ↑ Wijnen, H; Landman, A; Futcher, B (Jun 2002). "The G(1) cyclin Cln3 promotes cell cycle entry via the transcription factor Swi6.". Molecular and Cellular Biology. 22 (12): 4402–18. doi:10.1128/mcb.22.12.4402-4418.2002. PMC 133883
 . PMID 12024050.
. PMID 12024050. - ↑ Koch, C; Moll, T; Neuberg, M; Ahorn, H; Nasmyth, K (1993-09-17). "A role for the transcription factors Mbp1 and Swi4 in progression from G1 to S phase.". Science. 261 (5128): 1551–7. doi:10.1126/science.8372350. PMID 8372350.
- ↑ Dirick, L; Moll, T; Auer, H; Nasmyth, K (1992-06-11). "A central role for SWI6 in modulating cell cycle Start-specific transcription in yeast.". Nature. 357 (6378): 508–13. doi:10.1038/357508a0. PMID 1608451.
- ↑ Nasmyth, K; Dirick, L (1991-09-06). "The role of SWI4 and SWI6 in the activity of G1 cyclins in yeast.". Cell. 66 (5): 995–1013. doi:10.1016/0092-8674(91)90444-4. PMID 1832338.
- 1 2 Schwob, E; Böhm, T; Mendenhall, MD; Nasmyth, K (1994-10-21). "The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae.". Cell. 79 (2): 233–44. doi:10.1016/0092-8674(94)90193-7. PMID 7954792.
- ↑ Jorgensen, P; Nishikawa, JL; Breitkreutz, BJ; Tyers, M (2002-07-19). "Systematic identification of pathways that couple cell growth and division in yeast.". Science. 297 (5580): 395–400. doi:10.1126/science.1070850. PMID 12089449.
- ↑ de Bruin, RA; McDonald, WH; Kalashnikova, TI; Yates J, 3rd; Wittenberg, C (2004-06-25). "Cln3 activates G1-specific transcription via phosphorylation of the SBF bound repressor Whi5.". Cell. 117 (7): 887–98. doi:10.1016/j.cell.2004.05.025. PMID 15210110.
- ↑ Costanzo, M; Nishikawa, JL; Tang, X; Millman, JS; Schub, O; Breitkreuz, K; Dewar, D; Rupes, I; Andrews, B; Tyers, M (2004-06-25). "CDK activity antagonizes Whi5, an inhibitor of G1/S transcription in yeast.". Cell. 117 (7): 899–913. doi:10.1016/j.cell.2004.05.024. PMID 15210111.
- ↑ Koch, C; Schleiffer, A; Ammerer, G; Nasmyth, K (1996-01-15). "Switching transcription on and off during the yeast cell cycle: Cln/Cdc28 kinases activate bound transcription factor SBF (Swi4/Swi6) at start, whereas Clb/Cdc28 kinases displace it from the promoter in G2.". Genes & Development. 10 (2): 129–41. doi:10.1101/gad.10.2.129. PMID 8566747.
- ↑ Cosma, MP; Tanaka, T; Nasmyth, K (1999-04-30). "Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter.". Cell. 97 (3): 299–311. doi:10.1016/S0092-8674(00)80740-0. PMID 10319811.
- ↑ Ferrezuelo, F; Colomina, N; Futcher, B; Aldea, M (2010). "The transcriptional network activated by Cln3 cyclin at the G1-to-S transition of the yeast cell cycle.". Genome Biology. 11 (6): R67. doi:10.1186/gb-2010-11-6-r67. PMC 2911115
 . PMID 20573214.
. PMID 20573214. - ↑ Bean, JM; Siggia, ED; Cross, FR (Sep 2005). "High functional overlap between MluI cell-cycle box binding factor and Swi4/6 cell-cycle box binding factor in the G1/S transcriptional program in Saccharomyces cerevisiae.". Genetics. 171 (1): 49–61. doi:10.1534/genetics.105.044560. PMC 1456534
 . PMID 15965243.
. PMID 15965243. - ↑ Dirick, L; Nasmyth, K (1991-06-27). "Positive feedback in the activation of G1 cyclins in yeast.". Nature. 351 (6329): 754–7. doi:10.1038/351754a0. PMID 1829507.
- ↑ Cross, FR; Tinkelenberg, AH (1991-05-31). "A potential positive feedback loop controlling CLN1 and CLN2 gene expression at the start of the yeast cell cycle.". Cell. 65 (5): 875–83. doi:10.1016/0092-8674(91)90394-E. PMID 2040016.
- ↑ Stuart, D; Wittenberg, C (1995-11-15). "CLN3, not positive feedback, determines the timing of CLN2 transcription in cycling cells.". Genes & Development. 9 (22): 2780–94. doi:10.1101/gad.9.22.2780. PMID 7590253.
- ↑ Dirick, L; Böhm, T; Nasmyth, K (1995-10-02). "Roles and regulation of Cln-Cdc28 kinases at the start of the cell cycle of Saccharomyces cerevisiae.". The EMBO Journal. 14 (19): 4803–13. PMC 394578
 . PMID 7588610.
. PMID 7588610. - ↑ Skotheim, JM; Di Talia, S; Siggia, ED; Cross, FR (2008-07-17). "Positive feedback of G1 cyclins ensures coherent cell cycle entry.". Nature. 454 (7202): 291–6. doi:10.1038/nature07118. PMC 2606905
 . PMID 18633409.
. PMID 18633409. - ↑ Doncic, A; Falleur-Fettig, M; Skotheim, JM (2011-08-19). "Distinct interactions select and maintain a specific cell fate.". Molecular Cell. 43 (4): 528–39. doi:10.1016/j.molcel.2011.06.025. PMC 3160603
 . PMID 21855793.
. PMID 21855793. - ↑ Johnston, GC; Pringle, JR; Hartwell, LH (1977-03-01). "Coordination of growth with cell division in the yeast Saccharomyces cerevisiae.". Experimental Cell Research. 105 (1): 79–98. doi:10.1016/0014-4827(77)90154-9. PMID 320023.
- ↑ Hartwell, LH; Unger, MW (Nov 1977). "Unequal division in Saccharomyces cerevisiae and its implications for the control of cell division.". The Journal of Cell Biology. 75 (2 Pt 1): 422–35. doi:10.1083/jcb.75.2.422. PMC 2109951
 . PMID 400873.
. PMID 400873. - ↑ Di Talia, S; Skotheim, JM; Bean, JM; Siggia, ED; Cross, FR (2007-08-23). "The effects of molecular noise and size control on variability in the budding yeast cell cycle.". Nature. 448 (7156): 947–51. doi:10.1038/nature06072. PMID 17713537.
- ↑ Schneiderman, MH; Dewey, WC; Highfield, DP (Jul 1971). "Inhibition of DNA synthesis in synchronized Chinese hamster cells treated in G1 with cycloheximide.". Experimental Cell Research. 67 (1): 147–55. doi:10.1016/0014-4827(71)90630-6. PMID 5106077.
- ↑ Donachie, WD (1968-09-07). "Relationship between cell size and time of initiation of DNA replication.". Nature. 219 (5158): 1077–9. doi:10.1038/2191077a0. PMID 4876941.
- ↑ Elliott, SG; McLaughlin, CS (Sep 1978). "Rate of macromolecular synthesis through the cell cycle of the yeast Saccharomyces cerevisiae.". Proceedings of the National Academy of Sciences of the United States of America. 75 (9): 4384–8. doi:10.1073/pnas.75.9.4384. PMC 336119
 . PMID 360219.
. PMID 360219. - ↑ Popolo, L; Vanoni, M; Alberghina, L (Nov 1982). "Control of the yeast cell cycle by protein synthesis.". Experimental Cell Research. 142 (1): 69–78. doi:10.1016/0014-4827(82)90410-4. PMID 6754401.
- ↑ Moore, SA (1988-07-15). "Kinetic evidence for a critical rate of protein synthesis in the Saccharomyces cerevisiae yeast cell cycle.". The Journal of Biological Chemistry. 263 (20): 9674–81. PMID 3290211.
- ↑ Shilo, B; Riddle, VG; Pardee, AB (1979-10-15). "Protein turnover and cell-cycle initiation in yeast.". Experimental Cell Research. 123 (2): 221–7. doi:10.1016/0014-4827(79)90462-2. PMID 387426.
- ↑ Edgington, NP; Futcher, B (Dec 2001). "Relationship between the function and the location of G1 cyclins in S. cerevisiae.". Journal of Cell Science. 114 (Pt 24): 4599–611. PMID 11792824.
- ↑ Miller, ME; Cross, FR (Jan 2000). "Distinct subcellular localization patterns contribute to functional specificity of the Cln2 and Cln3 cyclins of Saccharomyces cerevisiae.". Molecular and Cellular Biology. 20 (2): 542–55. doi:10.1128/mcb.20.2.542-555.2000. PMC 85127
 . PMID 10611233.
. PMID 10611233. - ↑ Jorgensen, P; Edgington, NP; Schneider, BL; Rupes, I; Tyers, M; Futcher, B (Sep 2007). "The size of the nucleus increases as yeast cells grow.". Molecular Biology of the Cell. 18 (9): 3523–32. doi:10.1091/mbc.E06-10-0973. PMC 1951755
 . PMID 17596521.
. PMID 17596521. - ↑ Wang, H; Carey, LB; Cai, Y; Wijnen, H; Futcher, B (Sep 2009). "Recruitment of Cln3 cyclin to promoters controls cell cycle entry via histone deacetylase and other targets.". PLoS Biology. 7 (9): e1000189. doi:10.1371/journal.pbio.1000189. PMC 2730028
 . PMID 19823669.
. PMID 19823669. - ↑ Polymenis, M; Schmidt, EV (1997-10-01). "Coupling of cell division to cell growth by translational control of the G1 cyclin CLN3 in yeast.". Genes & Development. 11 (19): 2522–31. doi:10.1101/gad.11.19.2522. PMC 316559
 . PMID 9334317.
. PMID 9334317. - ↑ Vergés, E; Colomina, N; Garí, E; Gallego, C; Aldea, M (2007-06-08). "Cyclin Cln3 is retained at the ER and released by the J chaperone Ydj1 in late G1 to trigger cell cycle entry.". Molecular Cell. 26 (5): 649–62. doi:10.1016/j.molcel.2007.04.023. PMID 17560371.