Claudin
| PMP22_Claudin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | PMP22_Claudin | ||||||||
| Pfam | PF00822 | ||||||||
| Pfam clan | CL0375 | ||||||||
| InterPro | IPR004031 | ||||||||
| PROSITE | PDOC01045 | ||||||||
| TCDB | 1.H.1 | ||||||||
| OPM superfamily | 492 | ||||||||
| OPM protein | 4p79 | ||||||||
| |||||||||
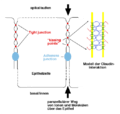
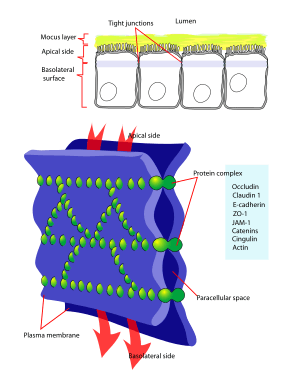
Claudins are a family of proteins that are the most important components of the tight junctions, where they establish the paracellular barrier that controls the flow of molecules in the intercellular space between the cells of an epithelium. They have four transmembrane domains, with the N-terminus and the C-terminus in the cytoplasm.
Structure
Claudins are small (20–27 kilodalton (kDa)) transmembrane proteins which are found in many organisms, ranging from nematodes to human beings, and are very similar in their structure, although this conservation is not observed on the genetic level. Claudins span the cellular membrane 4 times, with the N-terminal end and the C-terminal end both located in the cytoplasm, and two extracellular loops which show the highest degree of conservation. The first extracellular loop consists on average of 53 amino acids and the second one, being slightly smaller, of 24 amino acids. The N-terminal end is usually very short (4–10 amino acids), the C-terminal end varies in length from 21 to 63 and is necessary for the localisation of these proteins in the tight junctions.[1] It is suspected that the cysteines of individual or separate claudins form disulfide bonds. All human claudins (with the exception of Claudin 12) have domains that let them bind to PDZ domains of scaffold proteins.
History
Claudins were first named in 1998 by Japanese researchers Mikio Furuse and Shoichiro Tsukita at Kyoto University.[2] The name claudin comes from Latin word claudere ("to close"), suggesting the barrier role of these proteins.
A recent review discusses evidence regarding the structure and function of claudin family proteins using a systems approach to understand evidence generated by proteomics techniques.[3]
Genes
In humans, 24 members of the family have been described.
- CLDN1, CLDN2, CLDN3, CLDN4, CLDN5, CLDN6, CLDN7, CLDN8, CLDN9, CLDN10, CLDN11, CLDN12, CLDN13, CLDN14, CLDN15, CLDN16, CLDN17, CLDN18, CLDN19, CLDN20, CLDN21, CLDN22, CLDN23
See also
Additional images
References
- ↑ Rüffer C, Gerke V (May 2004). "The C-terminal cytoplasmic tail of claudins 1 and 5 but not its PDZ-binding motif is required for apical localization at epithelial and endothelial tight junctions". Eur. J. Cell Biol. 83 (4): 135–44. doi:10.1078/0171-9335-00366. PMID 15260435.
- ↑ Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S (June 1998). "Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin". J. Cell Biol. 141 (7): 1539–50. doi:10.1083/jcb.141.7.1539. PMC 2132999
 . PMID 9647647.
. PMID 9647647.
- ↑ Liu F, Koval M, Ranganathan S, Fanayan S, Hancock WS, Lundberg EK, Beavis RC, Lane L, Duek P, McQuade L, Kelleher NL, Baker MS (Dec 2015). "A systems proteomics view of the endogenous human claudin protein family". J Proteome Res. doi:10.1021/acs.jproteome.5b00769. PMID 26680015.