Classical complement pathway
The Classical Complement Pathway is a component of the innate immune system. The innate immune system serves as a constant non-specific defense against foreign pathogens including bacteria, viruses, fungi, and parasites.[1][2] Complement is a part of that system but it cannot act independently. Classical complement as discussed here relies on the actions of B cells to produce antibodies specific to different pathogens, as well as other components of the innate immune system such as Mast cells or Macrophages. It is an important aspect of our immune system and malfunction of complement can result in a compromised immune system. Complement deficiency has also been linked with autoimmune diseases and immunosuppression. Without complement we are unable to utilize the full strength of our immune system as macrophages and mast cells rely on it to phagocytose and release histamine respectively. While both of these cells are capable of functioning independently, the signal amplification that complement provides greatly increases their effectiveness.
Overview
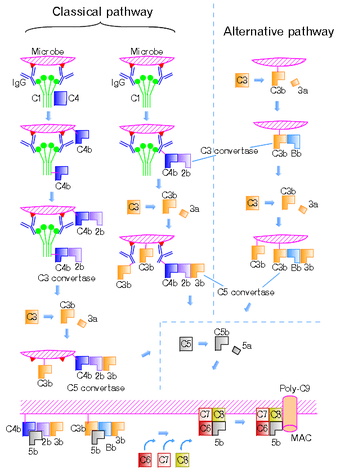
The classical complement pathway functions as a part of the complement system and leads to complement activation via the binding of antibodies to antigen. Both the IgM and IgG antibody isotypes are capable of initiating complement activation.[3] This initiation step then leads to the recruitment of other proteins in order to form the c3 convertase protein complex.[4] C3-convertase hydrolyzes C3 resulting in the complement cascade causing signal amplification and further complement activation.[5] The following steps in the pathway are similar to those initiated by the lectin pathway and alternative complement pathway. C3b binding results in further signal amplification and cleavage of C5, which leads to the formation of the Complement membrane attack complex, eventually forming a pore in the membrane of the target and inducing cell lysis and death. The classical pathway of complement activation is only one of three methods of activation, the others being the alternative complement pathway and the lectin pathway.[6]
Initiation and Steps Leading to C3-Convertase
The critical distinction between classical complement and other forms of complement is the role of antibodies to complement activation. Antibodies are essential to effective complement response in this pathway. The only antibodies capable of binding are IgG and IgM. It takes two units of IgG or one unit of IgM to trigger complement activation. IgM is thought of as more effective at activating complement (IgG4 cannot bind, but the other three IgG types can).
The next step in the classical complement pathway involves the binding of a protein known as c1q; c1q primarily binds to antibodies that have already attached themselves to antigen,[7] however, they can also bind directly to bacterial cell surfaces and viral envelopes.[8] In the absence of these targets however c1q exists as part of an inactive complex known simply as c1.

Following binding is the formation of the c1 complex (see figure 2) which then generates c1s esterase, an enzyme capable of cleaving inactive proteins c4 and c2 into their active forms (c4a and c4b, and c2a and c2b respectively).[9] The activated c2a and c4b fragments form a protein complex called c3 convertase (which is different from the one formed through the alternative complement pathway). C3 convertase in turn is capable of cleaving c3 into its respective fragments: c3b and c3a, which ultimately leads to signal amplification and the formation of the membrane attack complex.[10]
Signal Amplification and Formation of Membrane Attack Complex
One of the downstream effects of c3 convertase formation is signal amplification. In the presence of antibodies bound to antigen, the hydrolysis of c3 leads to the formation of further c3 convertase through the alternative pathway. This c3 convertase, while not structurally identical to the c3 convertase formed via the classical pathway, functions in a similar manner by cleaving c3 into c3a and c3b. This leads to a cascade of hydrolysis and greatly amplifies the initial signal.
The membrane attack complex forms after c3b has bound to the surface of the target pathogen and is able to bind to c4a, which was formed earlier, and an enzyme called c5 convertase. This enzyme, following the same pattern as the enzymes before it, cleaves c5 into c5a and c5b. Subsequent interactions between c5b, c6, c7, c8, and c9 form a protein complex known as the c5b-9 complex which is capable of forming pores on the surface of biological membranes and lysing foreign invaders.[7]
Clinical Significance
Because of its role in the innate immune system classical complement has been implicated in a number of pathogen related disorders. Complement is responsible for immune inflammatory response in adipose tissues which has been implicated in the development of obesity.[11] Obesity in turn results in an abnormally high level of complement activation via production of the c1 component of the classical pathway, which can lead to tissue inflammation and eventually insulin resistance, however the exact mechanisms that causes this is yet unknown.[11]
Immunotherapies have been developed to detect and destroy cells infected by the HIV virus via classical complement activation.[12] This process involves creating synthetic peptides that target conserved regions in HIV specific proteins and induce an antibody specific immune response through IgG antibodies. This is important for targeting the virus in its intracellular phase because the antibodies specific to the synthetic peptides can trigger the classical complement pathway and induce the death of HIV infected cells.
Classical complement activation has also been shown to combat Methicillin-resistant Staphylococcus aureus.[13] Certain variants of the IgM antibody were found to bind the Methicillin-resistant Staphylococcus aureus these IgM were found to be critical in complement activation through the classical pathway and subsequent destruction of the bacteria. Therapies that utilize classical complement activation have been shown to be effective in targeting and killing cancer cells and destroying tumors.[14] Tachyplesin, a small peptide, has been shown to exhibit these effects. When injected into target tissue encourages recruitment of c1q and activates downstream events, eventually leading to the formation of the c5b-9 complex which damages tumor cells, killing them.
See also
References
- ↑ Akira, Shizuo; Uematsu, Satoshi; Takeuchi, Osamu (2006-02-24). "Pathogen Recognition and Innate Immunity". Cell. 124 (4): 783–801. doi:10.1016/j.cell.2006.02.015. ISSN 0092-8674. PMID 16497588.
- ↑ Walport, M. J. (2001-04-05). "Complement. First of two parts". The New England Journal of Medicine. 344 (14): 1058–1066. doi:10.1056/NEJM200104053441406. ISSN 0028-4793. PMID 11287977.
- ↑ Jarius, Sven; Wildemann, Brigitte. "AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance". Nature Reviews Neurology. 6 (7): 383–392. doi:10.1038/nrneurol.2010.72.
- ↑ Kishore, Uday; Reid, Kenneth B. M (2000-08-01). "C1q: Structure, function, and receptors". Immunopharmacology. 49 (1–2): 159–170. doi:10.1016/S0162-3109(00)80301-X.
- ↑ Rus, Horea; Cudrici, Cornelia; Niculescu, Florin (2005-11-01). "The role of the complement system in innate immunity". Immunologic Research. 33 (2): 103–112. doi:10.1385/IR:33:2:103. ISSN 0257-277X.
- ↑ Esterbauer, H.; Krempler, F.; Oberkofler, H.; Patsch, W. (1999-08-01). "The complement system: a pathway linking host defence and adipocyte biology". European Journal of Clinical Investigation. 29 (8): 653–656. doi:10.1046/j.1365-2362.1999.00515.x. ISSN 0014-2972. PMID 10457146.
- 1 2 Rus, Horea; Cudrici, Cornelia; Niculescu, Florin (2005-11-01). "The role of the complement system in innate immunity". Immunologic Research. 33 (2): 103–112. doi:10.1385/IR:33:2:103. ISSN 0257-277X.
- ↑ Ahearn, Joseph M.; Fearon, Douglas T. (1989-01-01). Dixon, Frank J., ed. Structure and Function of the Complement Receptors, CR1 (CD35) and CR2 (CD21). 46. Academic Press. pp. 183–219. doi:10.1016/s0065-2776(08)60654-9.
- ↑ Krych-Goldberg, M.; Atkinson, J. P. (2001-04-01). "Structure-function relationships of complement receptor type 1". Immunological Reviews. 180: 112–122. doi:10.1034/j.1600-065x.2001.1800110.x. ISSN 0105-2896. PMID 11414353.
- ↑ Rutemark, Christian; Alicot, Elisabeth; Bergman, Anna; Ma, Minghe; Getahun, Andrew; Ellmerich, Stephan; Carroll, Michael C.; Heyman, Birgitta (2011-10-25). "Requirement for complement in antibody responses is not explained by the classic pathway activator IgM". Proceedings of the National Academy of Sciences. 108 (43): E934–E942. doi:10.1073/pnas.1109831108. ISSN 0027-8424. PMC 3203774
 . PMID 21987785.
. PMID 21987785. - 1 2 Zhang, Jinhui; Wright, Wendy; Bernlohr, David A.; Cushman, Samuel W.; Chen, Xiaoli (2007-05-01). "Alterations of the classic pathway of complement in adipose tissue of obesity and insulin resistance". American Journal of Physiology - Endocrinology and Metabolism. 292 (5): E1433–E1440. doi:10.1152/ajpendo.00664.2006. ISSN 0193-1849. PMID 17244723.
- ↑ Pleguezuelos, Olga; Stoloff, Gregory A; Caparrós-Wanderley, Wilson (2013-04-04). "Synthetic immunotherapy induces HIV virus specific Th1 cytotoxic response and death of an HIV-1 infected human cell line through classic complement activation". Virology Journal. 10 (1). doi:10.1186/1743-422x-10-107.
- ↑ An, Jingang; Li, Zhengxiao; Dong, Yingying; Wu, Jiawen; Ren, Jianwen (2015-05-22). "Complement activation contributes to the anti-methicillin-resistant Staphylococcus aureus effect of natural anti-keratin antibody". Biochemical and Biophysical Research Communications. 461 (1): 142–147. doi:10.1016/j.bbrc.2015.03.182.
- ↑ Chen, Jinguo; Xu, Xue-Ming; Underhill, Charles B.; Yang, Shanmin; Wang, Luping; Chen, Yixin; Hong, Shuigen; Creswell, Karen; Zhang, Lurong (2005-06-01). "Tachyplesin Activates the Classic Complement Pathway to Kill Tumor Cells". Cancer Research. 65 (11): 4614–4622. doi:10.1158/0008-5472.CAN-04-2253. ISSN 0008-5472. PMID 15930279.