Chromite
| Chromite | |
|---|---|
 | |
| General | |
| Category |
Oxide minerals Spinel group Spinel structural group |
| Formula (repeating unit) | (Fe, Mg)Cr2O4 |
| Strunz classification | 04.BB.05 |
| Crystal system | Isometric |
| Space group |
Isometric hexoctahedral H-M symbol: (4/m3 2/m) Space group: F d3m |
| Unit cell | a = 8.344 Å; Z = 8 |
| Identification | |
| Color | Black to brownish black; brown to brownish black on thin edges in transmitted light |
| Crystal habit | Octahedral rare; massive to granular |
| Twinning | Spinel law on {1ll} |
| Cleavage | None, parting may develop along {111} |
| Fracture | Uneven |
| Tenacity | Brittle |
| Mohs scale hardness | 5.5 |
| Luster | Submetallic |
| Streak | Brown |
| Diaphaneity | Translucent to opaque. |
| Specific gravity | 4.5 - 4.8 |
| Optical properties | Isotropic |
| Refractive index | n = 2.08-2.16 |
| Other characteristics | Weakly magnetic |
| References | [1][2][3][4] |
Chromite is an iron chromium oxide: FeCr2O4. It is an oxide mineral belonging to the spinel group. Magnesium can substitute for iron in variable amounts as it forms a solid solution with magnesiochromite (MgCr2O4);[5] substitution of aluminium occurs leading to hercynite (FeAl2O4).[6]
It is by far the most industrially important mineral for the production of metallic chromium, used as an alloying ingredient in stainless and tool steels.
Occurrence

Chromite is found as orthocumulate lenses of chromitite in peridotite from the Earth's mantle. It also occurs in layered ultramafic intrusive rocks.[7] In addition, it is found in metamorphic rocks such as some serpentinites. Ore deposits of chromite form as early magmatic differentiates. It is commonly associated with olivine, magnetite, serpentine, and corundum. The vast Bushveld igneous complex of South Africa is a large layered mafic to ultramafic igneous body with some layers consisting of 90% chromite making the rare rock type, chromitite.[8] The Stillwater igneous complex in Montana also contains significant chromite.[2]
Uses
The only ores of chromium are the minerals chromite and magnesiochromite. Most of the time, economic geology names chromite the whole chromite-magnesiochromite series: FeCr2O4, (Fe,Mg)Cr2O4, (Mg,Fe)Cr2O4 and MgCr2O4.[4] The two main products of chromite refining are ferrochromium and metallic chromium; for those products the ore smelter process differs considerably. For the production of ferrochromium the chromite ore (FeCr2O4) is reduced with either aluminium or silicon in an aluminothermic reaction and for the production of pure chromium the iron has to be separated from the chromium in a two step roasting and leaching process.[9] Chromite is also used as a refractory material, because it has a high heat stability.[10]
The chromium extracted from chromite is used in chrome plating and alloying for production of corrosion resistant superalloys, nichrome, and stainless steel. Chromium is used as a pigment for glass, glazes, and paint, and as an oxidizing agent for tanning leather.[11] It is also sometimes used as a gemstone.[12]
Mining
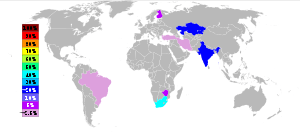
In 2002 14,600,000 metric tons of chromite were mined. The largest producers were South Africa (44%), India (18%),[13] Kazakhstan (16%), Zimbabwe (5%), Finland (4%), Iran (4%), and Brazil (2%), with several other countries producing the rest of less than 10% of the world production.[14][15]
Minor production
Afghanistan has significant deposits of high grade chromite ore, which is mined illegally in Khost Province and then smuggled out of the country to Pakistan.[16]
In Pakistan, chromite is mined from the ultramafic rocks in mainly the Muslimbagh killa Saifullah District and Khanozai area of Pishin District of Balochistan. Most of the chromite is of metallurgical grade with Cr2O3 averaging 54% and a chrome to iron ratio of 2.6:1.
Recently, the biggest user of chromite ore has been China, importing large quantities from South Africa, Pakistan, and other countries. The concentrate is used to make ferrochrome, which is in turn used to make stainless steel and some other alloys.[17]
In April 2010 the Government of Ontario announced[18] that they would be opening up a large chromite deposit to development in the northern part of Ontario known as the Ring of Fire. This plan has since been suspended.[19]
References
- ↑ Handbook of Mineralogy
- 1 2 Klein, Corneis and Cornelius S. Hurlbut, Manual of Mineralogy, Wiley, 20th ed., pp. 312–313. ISBN 0-471-80580-7.
- ↑ Webmineral data
- 1 2 Mindat.org
- ↑ http://www.mindat.org/min-8675.html Mindat
- ↑ http://www.mindat.org/min-8674.html Mindat
- ↑ Gu, F; Wills, B (1988). "Chromite- mineralogy and processing". Minerals Engineering. 1 (3): 235. doi:10.1016/0892-6875(88)90045-3.
- ↑ Guilbert, John M., and Park, Charles F., Jr. (1986) The Geology of Ore Deposits, Freeman, ISBN 0-7167-1456-6
- ↑ Papp, John F.; Lipin Bruce R. (2006). "Chromite". Industrial Minerals & Rocks: Commodities, Markets, and Uses (7th ed.). SME. ISBN 978-0-87335-233-8.
- ↑ Routschka, Gerald (2008). Pocket Manual Refractory Materials: Structure - Properties - Verification. Vulkan-Verlag. ISBN 978-3-8027-3158-7.
- ↑ http://www.mineralszone.com/minerals/chromite.html
- ↑ Tables of Gemstone Identification By Roger Dedeyne, Ivo Quintens, p.189
- ↑ "Chromites of India" (PDF).
- ↑ Papp, John F. "Mineral Commodity Summary 2006: Chromium" (PDF). United States Geological Survey. Retrieved 2009-02-24.
- ↑ Papp, John F. "Minerals Yearbook 2006: Chromium" (PDF). United States Geological Survey. Retrieved 2009-02-24.
- ↑ Bowley, Graham (2012-09-08). "Afghans Wary as Efforts Pick Up to Tap Mineral Riches". The New York Times.
- ↑ "How Products are Made, Vol. 1". Retrieved 29 Dec 2010.
- ↑ "YouTube - premierofontario's Channel". YouTube. Retrieved 2010-04-13.
- ↑ "Cliffs suspends Ring of Fire project in northern Ontario". CBC News. Retrieved 2013-11-28.
External links
| Wikimedia Commons has media related to Chromite. |