Chemical eye injury
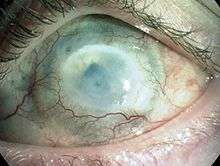
Chemical eye injury or chemical burns to the eye are due to either an acidic or alkali substance getting in the eye.[1] Alkalis are typically worse than acidic burns.[2] Mild burns will produce conjunctivitis while more severe burns may cause the cornea to turn white.[2] Litmus paper is an easy way to rule out the diagnosis by verifying that the pH is within the normal range of 7.0—7.2.[1] Large volumes of irrigation is the treatment of choice and should continue until the pH is 6—8.[2] Local anaesthetic eye drops can be used to decrease the pain.[2]
Epidemiology
In the United States, chemical eye injuries most commonly occur among working-age adults.[3] A 2016 analysis of emergency department visits from 2010-2013 reported over 36,000 visits annually for chemical burns to the eye, with a median age at presentation of 32 years.[4] By individual year of age, 1- and 2-year-old children have the highest incidence of these injuries, with rates approximately 50% higher than the highest-risk adult group (25 years), and 13 times higher than the rate among 7-year-olds.[4]
References
- 1 2 Zentani A, Burslem J (December 2009). "Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. BET 4: use of litmus paper in chemical eye injury". Emerg Med J. 26 (12): 887. doi:10.1136/emj.2009.086124. PMID 19934140.
- 1 2 3 4 Hodge C, Lawless M (July 2008). "Ocular emergencies". Aust Fam Physician. 37 (7): 506–9. PMID 18592066.
- ↑ Saini JS, Sharma A (February 1993). "Ocular chemical burns-clinical and demographic profile.". Burns. 19 (1): 67–69. doi:10.1016/0305-4179(93)90104-G.
- 1 2 Haring RS, Sheffield ID, Channa R, Canner JK, Schneider EB (August 2016). "Epidemiologic Trends of Chemical Ocular Burns in the United States.". JAMA Ophthalmology. (epub ahead of print). doi:10.1001/jamaophthalmol.2016.2645. PMID 27490908.